Exercise-2.2 Page: 18 ( Is Matter Around Us Pure )
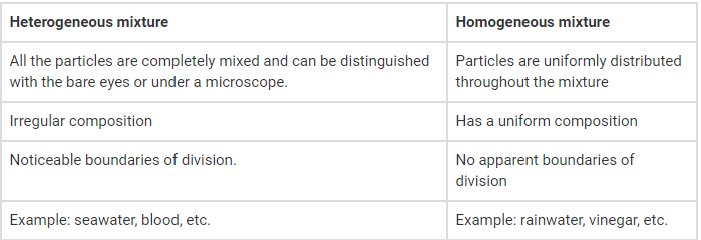
1. Differentiate between homogenous and heterogeneous mixtures with examples.
Solution:
The following are the differences between heterogeneous and homogenous mixtures.
2. How are sol, solution and suspension different from each other?
Solution:

3. To make a saturated solution, 36g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Solution:
Mass of solute (NaCl) = 36 g
Mass of solvent (H2O) = 100 g
Mass of solution (NaCl + H2O) = 136 g
Concentration = Mass of solute/Mass of solution x 100
Concentration = 36/136 x 100 = 26.47%
Hence, the concentration of the solution is 26.47%
Category: CBSE Class 9th Science, Class 9th
Subscribe
Login
0 Comments
Oldest



