www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Engineering post, Career Guidelines , English Speaking Trick , How to crack interview and lots more. ( Class 11 Physics Chapter 11 Thermal Properties of matter )
NCERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of matter
NCERT Solutions for Class 11 Physics Chapter 11 Thermal Properties of matter:
| Section Name | Topic Name |
| 11 | Thermal Properties of matter |
| 11.1 | Introduction |
| 11.2 | Temperature and heat |
| 11.3 | Measurement of temperature |
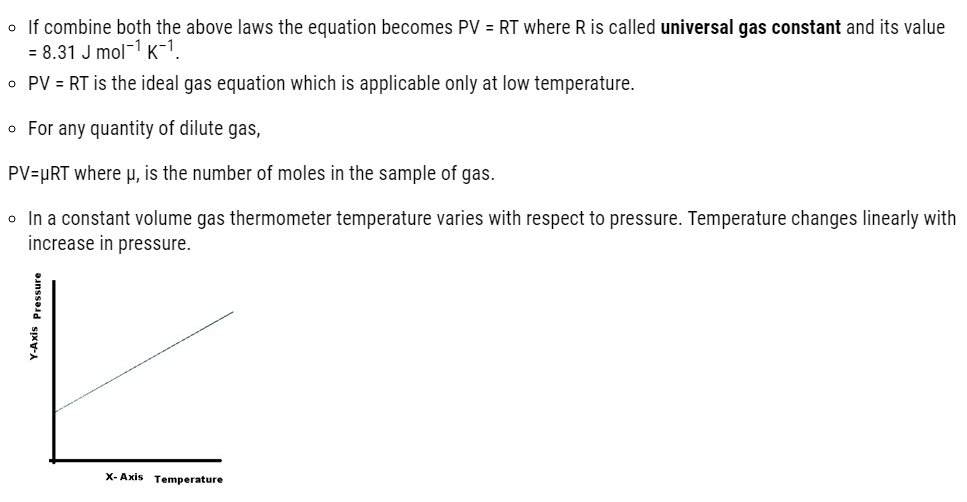
| 11.4 | Ideal-gas equation and absolute temperature |
| 11.5 | Thermal expansion |
| 11.6 | Specific heat capacity |
| 11.7 | Calorimetry |
| 11.8 | Change of state |
| 11.9 | Heat transfer |
| 11.10 | Newton’s law of cooling |
Introduction
You might have noticed that youfeel hotter on a sunny afternoon as compared to a windy night. This is because of the difference in temperatures. Temperature is very high in the afternoon as compared to night. This chapter basically gives us the
Examples: information about thermal properties of matter where we will study about the properties of different substances by virtue of heat / heat transfer.
In simple terms, we can say that when temperature is more heat is more and when temperature is less heat is less.
Hot Sunny day (Temperature is more)and ice cold water (Temperature is less).
Temperature and Heat
Temperature is defined as the measure of degree of hotness or coldness of a body.
Example:-
- A cup of hotsoup or a scoop of Ice-cream.

After some time we will see this hot cup of coffee will become cold as there will be transfer of heat.
The S.I Unit of Heat is joule (J) and some of the commonly used units are: calorie and kilocalorie
Relation between Joule and Calorie
1calorie=4.18 Joules
1kilocalorie = 1000 calories
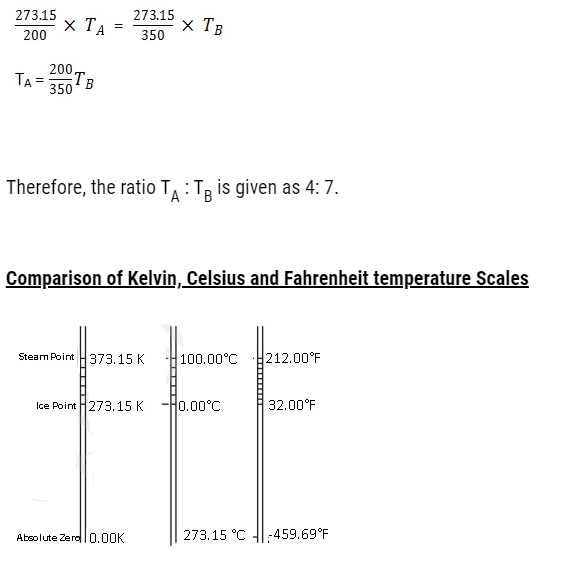
The S.I. Unit of Temperature is Kelvin (K) and some of the commonly used unitsare:Fahrenheit (°F) and Celsius (°C)
Measurement of Temperature
Temperature is measured with the help of thermometer. Mercury and Alcohol are commonly used liquids in the liquid-in-glass thermometers.
- To construct a thermometer two fixed points are to be chosen as a reference points. These fixed points are known asfreezing(ice point) and boiling point(steam point). The water freezes and boils at these two points under standard pressure.
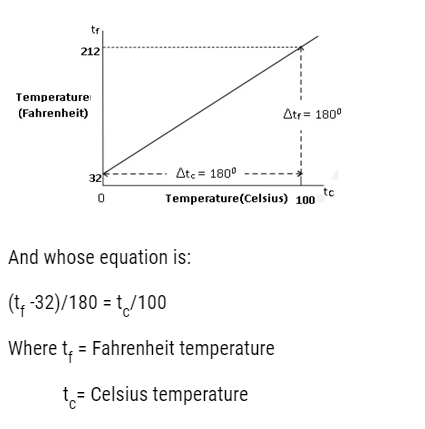
- The ice and steam point in Fahrenheit Temperature scale are 32°F and 212 °F resp.It has 180 equal intervals between two reference points.
- On Celsius Scale values are 0°C and 100°C for ice and steam point resp. It has 100 equal intervals between two reference points.

Ideal-gas Equation and Absolute Temperature
A thermometer that uses any gas, however, gives the same readings regardless of which gas is used because all gases have same expansion at low temperature.
Variables that describe the behaviour of gas are:-
- Quantity(mass)
- Pressure
- Volume
- Temperature i.e. (P,V,T) where (T = t + 273.15; t is the temperature in °C)
Gases which have low density obey certain laws: –
1.Boyle’s Law– PV = constant(when temperature T is constant)
2.Charles’ Law– V/T = constant (when pressure P is constant)

Problem:The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales?
Solution: Celsius and Fahrenheit scales are related as
TF = (9/5)TC + 32 … (ii)
For neon:
= 24.57 K
= 24.57 – 273.15 = –248.58°C
TF = (9/5) TC + 32
=9/5(-248.58) +32
=415.44 oF
For carbon dioxide:
= 216.55 K
= 216.55 – 273.15 = –56.60°C
TF = (9/5)TC + 32
=9/5(-56.60) +32
= -69.88 oC
Problem:Two absolute scales A and B have triple points of water defined to be 200 A and 350 B. What is the relation between TA and TB?
Solution:
Triple point of water on absolute scale A, T1 = 200 A
Triple point of water on absolute scale B, T2 = 350 B
Triple point of water on Kelvin scale, Tk= 273.15 K
The temperature 273.15 K on Kelvin scale is equivalent to 200 A on absolute scale A.
T1 = Tk
200 A = 273.15 K
Therefore,
A = 273.15/200
The temperature 273.15 K on Kelvin scale is equivalent to 350 B on absolute scale B.
T2 = Tk
350 B = 273.15
B= 273.15/350
TA is triple point of water on scale A. TB is triple point of water on scale B
Therefore,
Thermal Expansion
- Thermal expansion is the phenomenon of increase in dimensions of a body due to increase in its temperature.
Examples of Thermal Expansion
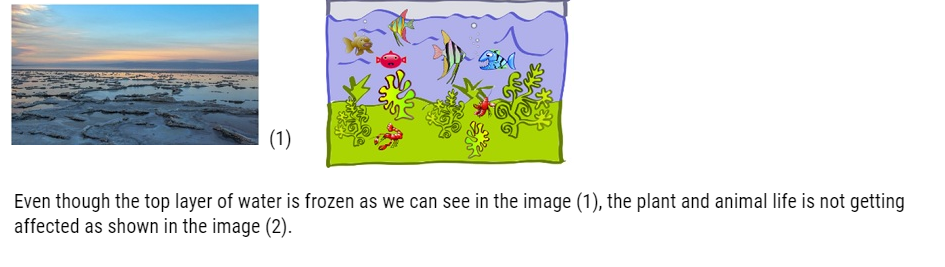
- The water is cold at the top of the lake because it expands and becomes less dense. So when this water freezes it insulates the water below it from the outside which means cold air is like a blanket. It is because of this property many fish can survive in the winter.

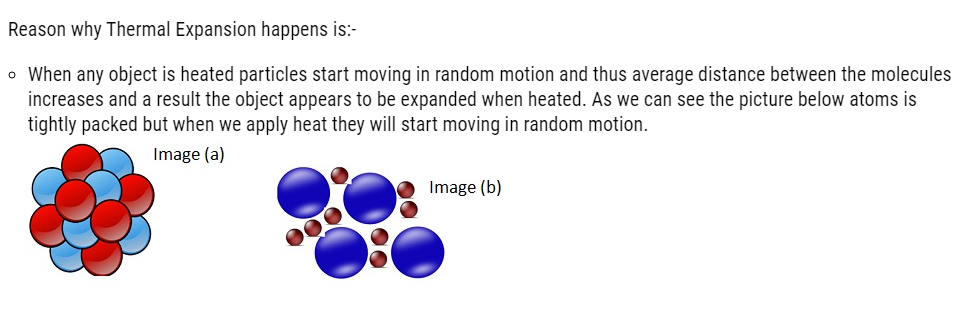
As we can see in the Image (a) molecules are very tightly packed but when heated the molecules start moving apart in random motion, which can be seen in Image (b).
- When an object is cooled it contracts which is referred as negative thermal expansion.
Types of Thermal Expansion
- Linear Expansion :- The expansion in length
- Area Expansion :- The expansion in area
- Volume Expansion :- The expansion in volume
Linear Expansion
Linear Expansion means expansion in length due to increase in temperature. Linear expansion means fractional change in length i.e. how the length is changing with respect to original length.



- It is denoted by αa
- It is characteristic of the substance and it varies with temperature.
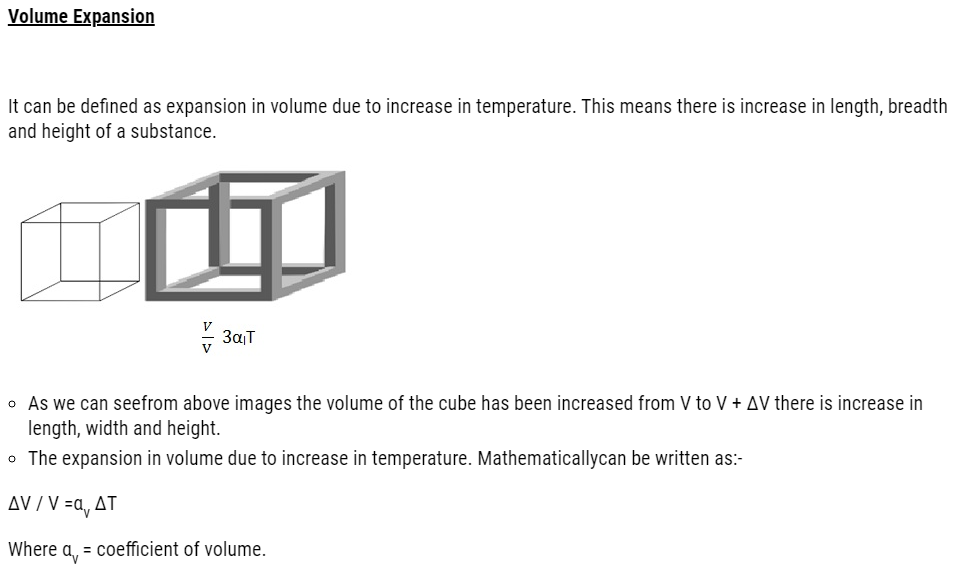
Coefficient of Volume can be defined as degree of volume expansion divided by change in temperature.
- It is denoted by αv.
- It is characteristic of the substance
- It varies with temperature.
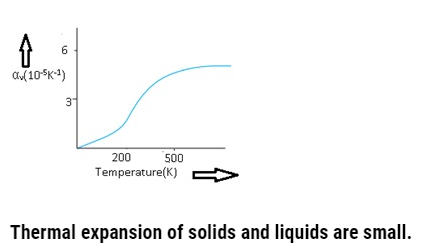
If graph is plotted between αv and temperature,then initially αv is a changing linearly then it varies non-linearly and at higher temperatures and then it becomes constant.
Coefficient of volume expansion of copper as a function of temperature-
Anomalous Behaviour of Water
- Water shows some exceptional behaviour that is when it is heated at 0°C, it contracts instead of expandingand it happens till it reaches 4 °C. The volume of a given amount of water is minimumat 4 °C therefore its density is maximum(Refer the Fig). After 4 °C water starts expanding. Below 4 °C, the volume increases, and therefore the density decreases. This means water has maximum density at 4 °C.
- Advantages of Anomalous behaviour of Water
- Because of this property of water in lakes and ponds freeze only at the top layer and at the bottom it does not, butif the water freezes at the bottom also then animal and plant life would not be possible.
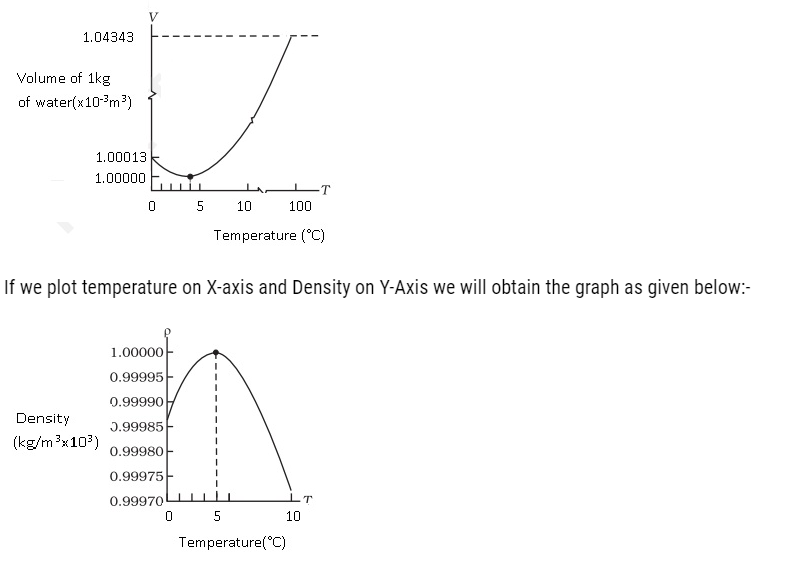
The information we get from the above graph means that the density increases as its temperature rises from 0°C to 4 °C and density decreases after 4°C.

By solving we get ΔV= 3l2Δl(we are neglecting (Δl)2 and (Δl)3as they are very small as to compared to l.
Therefore, Δ V = (3V Δl)/l
=3VαlΔT
Which gives αv = 3αl the relation between coefficient of volume expansion and coefficient of linear expansion.
Thermal stress: ( Thermal Properties of Matter )
- Mechanical stress induced by a body when some or all of its parts are not free to expand or contract in response to change in temperature.
- When an object is heated or cooled either it expands or it contracts but if for some reason if the object is not allowed to expand to contract under that case mechanical stress is induced in the body which is known as Thermal Stress.
- Example :-
While designing structures like concrete highways gaps are left which are filled by some flexible material so that concrete is allowed to expand or contract.
Heat Capacity ( Thermal Properties of Matter )
The change in temperature of a substance, when a given quantity of heat is absorbed or rejected by substance is characterised by a quantity called the heat capacity of that substance.
- It is denoted by S.
- It is given as S = ΔQ/ ΔT
Where ΔQ = amount of heat supplied to the substance and T to T + ΔT change in its temperature.


Molar specific heat capacity: –
- Heat capacity per mole of the substance is the defined as the amount of heat (in moles) absorbed or rejected(instead of mass m in kg) by the substance to change its temperature by one unit.
Mathematically can be written as:-
C = S/ μ= ΔQ / μ ΔT
Where
- μ= amount of substance in moles
- C = molar specific heat capacity of the substance.
- ΔQ = amount of heat absorbed or rejected by a substance.
- ΔT = temperature change
It depends on the nature of the substance and its temperature. The SI unit of molar specific heat capacity is Jmol–1 K–1
Molar specific heat capacity (Cp):-
- If the gas is held under constant pressure during the heat transfer, then the corresponding molar specific heat capacity is called molar specific heat capacity at constant pressure (Cp).
Molar specific heat capacity (Cv):-
- If the volume of the gas is maintained during the heat transfer, then the corresponding molar specific heat capacity is called molar specific heat capacity at constant volume (Cv).
- Water has highest specific heat of capacity because of which it is used as a coolant in automobile radiators and in hot water bags.

Solution:- Mass of the metal, m = 0.20 kg = 200 g
Initial temperature of the metal, T1 = 150°C
Final temperature of the metal, T2 = 40°C
Calorimeter has water equivalent of mass, m’ = 0.025 kg = 25 g
Volume of water, V = 150 cm3
Mass (M) of water at temperature T = 27°C: 150 × 1 = 150 g
Fall in the temperature of the metal:
ΔT = T1 – T2 = 150 – 40 = 110°C
Specific heat of water, Cw = 4.186 J/g/°K
Specific heat of the metal = C
Heat lost by the metal, θ = mCΔT … (i)
Rise in the temperature of the water and calorimeter system:
ΔT = 40 – 27 = 13°C
Heat gained by the water and calorimeter system:
Δθ’’ = m1 CwΔT’
= (M + m′) Cw ΔT’ … (ii)
Heat lost by the metal = Heat gained by the water and calorimeter system
mCΔT = (M + m’) Cw ΔT’
200 × C × 110 = (150 + 25) × 4.186 × 13

Calorimetry
Calorimetry is made up of 2 words:-
- Calorie which means heat andmetry means measurement.Therefore Calorimetry means measurement of heat.
- Calorimetry is defined as heat transfers from a body at a higher temperature to a body at a lower temperature provided there is no loss of heat to the atmosphere.
- Principle of Calorimetry is heat lost by one body is equal to the heat gained by another body.
- The Device which measures Calorimetry is known as Calorimeter.
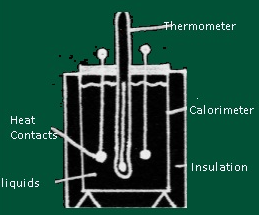
- Description of Calorimeter
- A calorimeter consists of metallic vessel and a stirrer both are made of same material (copper or aluminium) and the vessel is kept in a wooden jacket so that there is no heat loss .A mercury thermometer can be inserted through a small opening in the outer jacket.
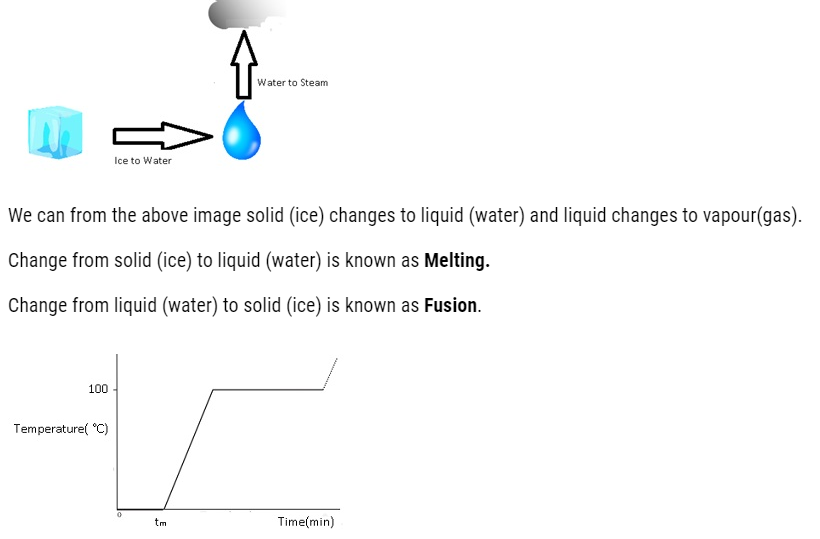
Change of State
The transition from either solid to liquid or gas and gas to either liquid or solid is termed as change of state.
Thermal Equilibrium: – At this state there is no loss or gain of heat takes place.
The temperature at which the solid and the liquid states of the substance are in thermal equilibrium with each other is called its melting point.

Regelation:-
- Regelation can be defined as phenomenon in which the freezing point of water is lowered by the application of pressure.
Example:-

Cause of regelation:-
- If we have a block of ice and a copper wire pulled by two masses if we will observe that copper wire can pass through ice block this is because copper is good conductor of heat so as it passes through the ice it gets refreeze as the copper will generate heat and this heat will pass quickly to the ice below and it starts melting because there is increase in pressure which lowers temperature as a result the wire will move through the ice. This happens because of regelation.
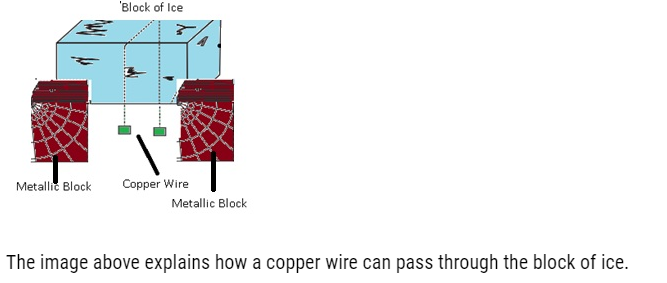
The image above explains how a copper wire can pass through the block of ice.
Vaporisation: – Transition from liquid to vapour.
- The change of state from liquid to vapour (or gas) is called vaporisation.


Related link you must like:-
Study material for Competition Exam
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]






