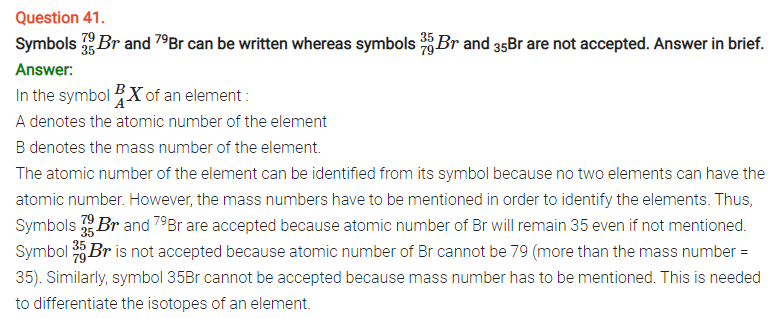
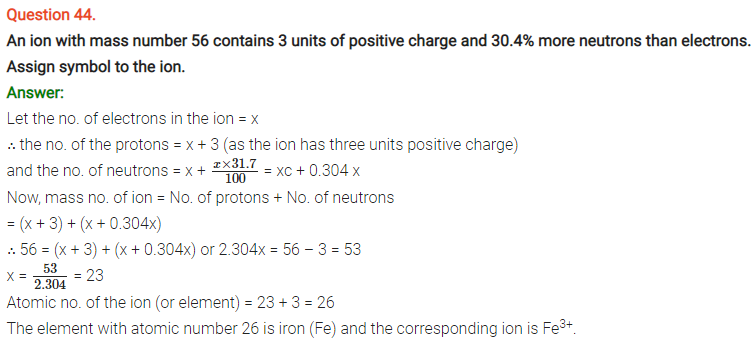
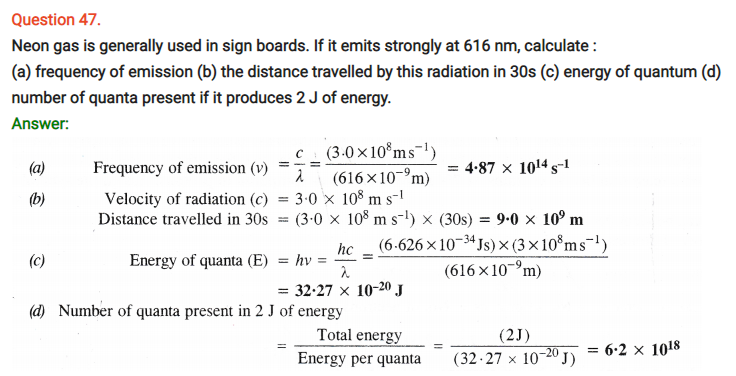
NCERT Solutions for Class 11 Chemistry Chapter 2 Structure of Atom
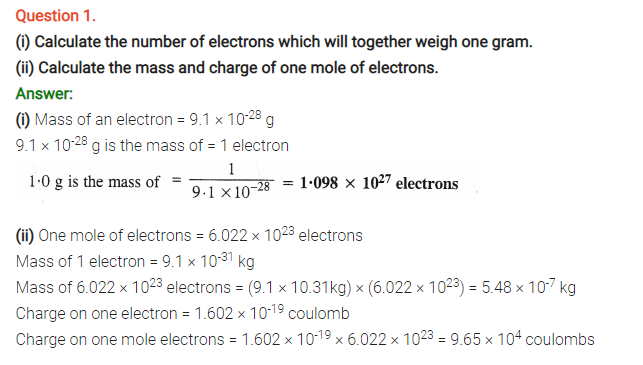
Question 2.
(i) Calculate the total number of electrons present in one mole of methane.
(ii) Find (a) the total number and (b) the total mass of neutrons in 7 mg of 14C. (Assume that mass of a neutron = 1.675 × 10-27kg).
(iii) Find (a) the total number and (b) the total mass of protons in 34 mg of NH3 at STP.
Will the answer change if the temperature and pressure are changed ?
Answer:
(i) One mole of methane (CH4) has molecules = 6.022 × 1023
No. of electrons present in one molecule of CH4 = 6 + 4 = 10
No. of electrons present in 6.022 × 1023 molecules of CH4 = 6.022 × 1023 × 10
= 6.022 × 1024 electrons
(ii)Step I. Calculation of total number of carbon atoms
Gram atomic mass of carbon (C-14) = 14 g = 14 × 103 mg
14 × 103 mg of carbon (C-14) have atoms = 6.022 × 1023
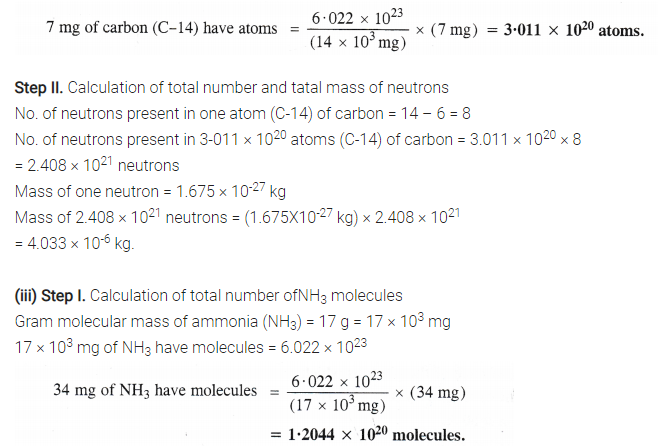
Step II. Calculation of total number and mass of protons
No. of protons present in one molecule of NH3 = 7 + 3 = 10 .
No. of protons present in 12.044 × 1020 molecules of NH3 = 12.044 × 1020 × 10
= 1.2044 × 1022 protons
Mass of one proton = 1.67 × 10-27 kg
Mass of 1.2044 × 1022 protons = (1.67 × 10-27 kg) × 1.2044 × 1022
= 2.01 × 10-5 kg.
No, the answer will not change upon changing the temperature and pressure because only the number of protons and mass of protons are involved.






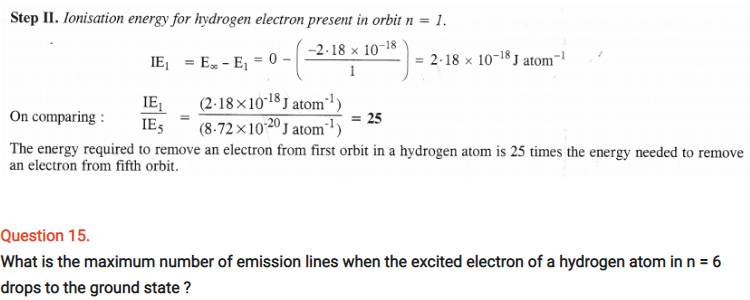
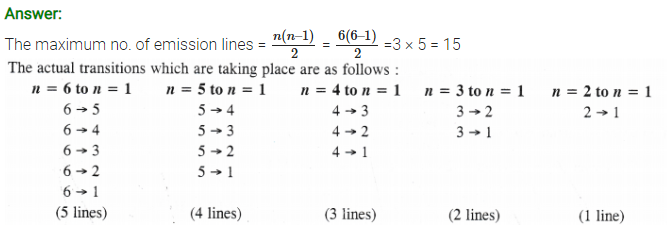

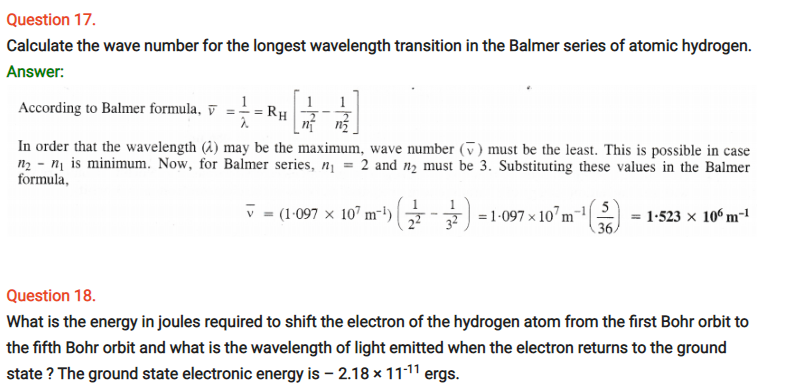
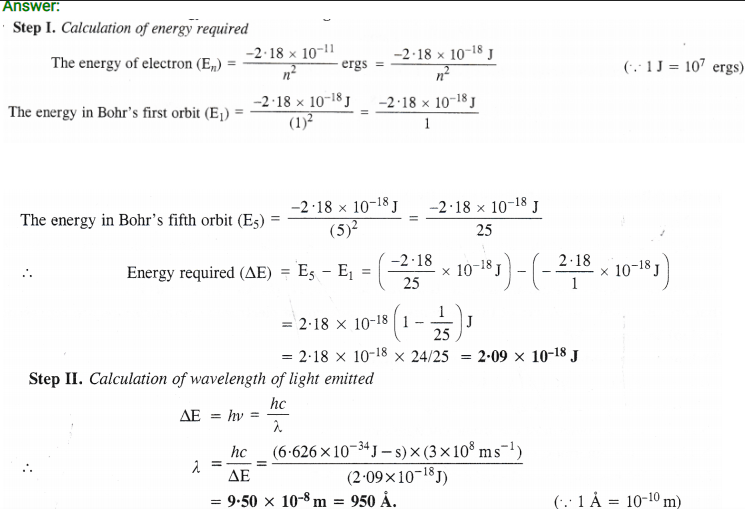



Question 25.
An electron is in one of the 3d orbitals. Give the possible values of n, l and nil for the electron.
Answer:
For electron in 3d orbital, n = 3, l = 2, mi = -2, -1, 0, +1, +2.
Question 26.
An atom of an element contains 29 electrons and 35 neutrons. Deduce (i) the number of protons and (ii) the electronic configuration of the element.
Answer:
No. of protons in a neutral atom = No. of electrons = 29
Electronic configuration = 1s2 2s2 2p6 3s2 3p6 3d10 4s1.
Question 27.
Give the number of electrons in the species : H2+, H2 and 02+.
Answer:
H2+ = one ; H2 = two ; 02+ = 15
Question 28.
(i) An atomic orbital has n = 3. What are the possible values of l and ml ?
(ii) List the quantum numbers ml and l of electron in 3rd orbital.
(iii) Which of the following orbitals are possible ?
1p, 2s, 2p and 3f.
Answer:
(i) For n = 3; l = 0, 1 and 2.
For l = 0 ; ml = 0
For l = 1; ml = +1, 0, -1
For l = 2 ; ml = +2, +1,0, +1, + 2
(ii) For an electron in 3rd orbital ; n = 3; l = 2 ; ml can have any of the values -2, -1, 0,
+ 1, +2.
(iii) 1p and 3f orbitals are not possible.
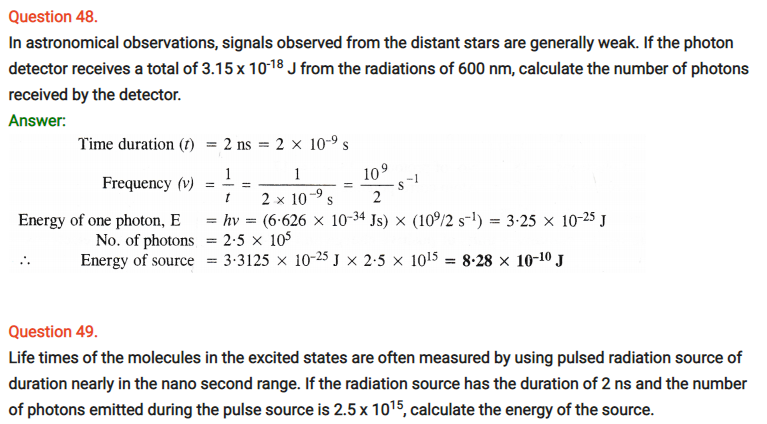
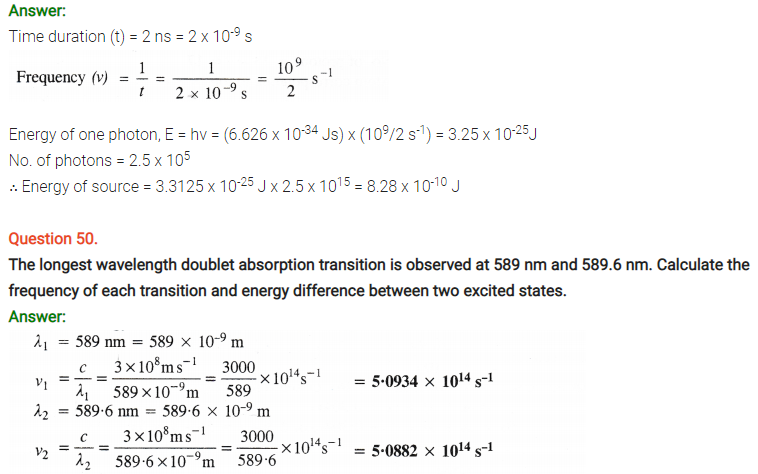
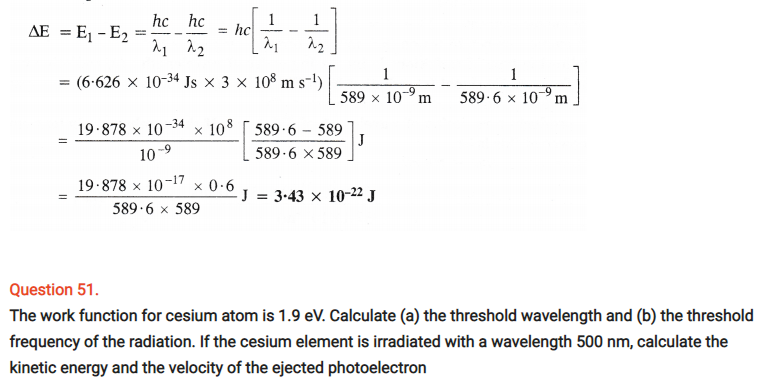
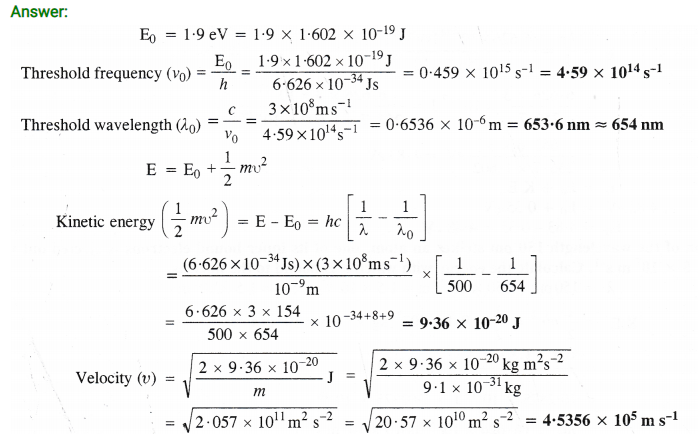
Question 29.
Using s, p and d notations, describe the orbitals with follow ing quantum numbers :
(a) n = 1, l = 0
(b) n = 4, l = 3
(c) n = 3, l = 1
(d) n = 4, l = 2
Answer:
(a) 1s orbital
(b) 4f orbital
(c) 3p orbital
(d) 4d orbital
Question 30.
From the following sets of quantum numbers, state which are possible. Explain why the others are not possible.
(i) n = 0, l = 0, ml = 0, ms = +1/2
(ii) n = 1, l = 0, ml = 0, ms – – 1/2
(iii) n = 1, l = 1, ml = 0, ms= +1/2
(iv) n = 1, l = 0, ml = +1, ms= +1/2
(v) n = 3, l = 3, ml = -3, ms = +1/2
(vi) n = 3, l = 1, ml = 0, ms= +1/2
Answer:
(i) The set of quantum numbers is not possible because the minimum value of n can be 1 and not zero.
(ii) The set of quantum numbers is possible.
(iii) The set of quantum numbers is not possible because, for n = 1, l can not be equal to 1. It can have 0 value.
(iv) The set of quantum numbers is not possible because for l = 0. mt cannot be + 1. It must be zero.
(v) The set of quantum numbers is not possible because, for n = 3, l ≠ 3.
(vi) The set of quantum numbers is possible.
Question 31.
How many electrons in an atom may have the following quantum numbers?
(a) n = 4 ; ms = -1/2
(b) n = 3, l = 0.
Answer:
(a) For n = 4
Total number of electrons = 2n2 = 2 × 16 = 32
Half out of these will have ms = —1/2
∴ Total electrons with ms (-1/2) = 16
(b) For n = 3
l= 0 ; ml = 0, ms +1/2, -1/2 (two e–)
Question 32.
Show that the circumference of the Bohr orbit for the hydrogen atom is an integral multiple of the de Broglie wavelength associated with the electron revolving around the orbit.
Answer:
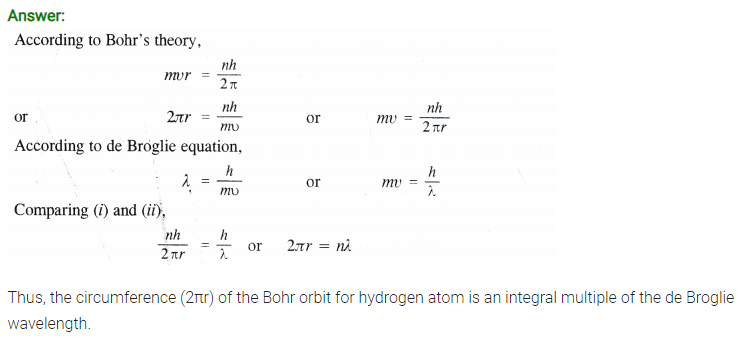

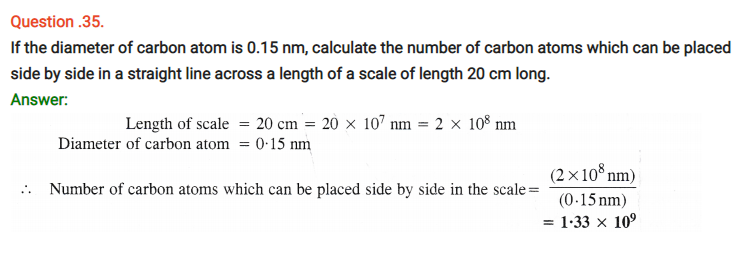
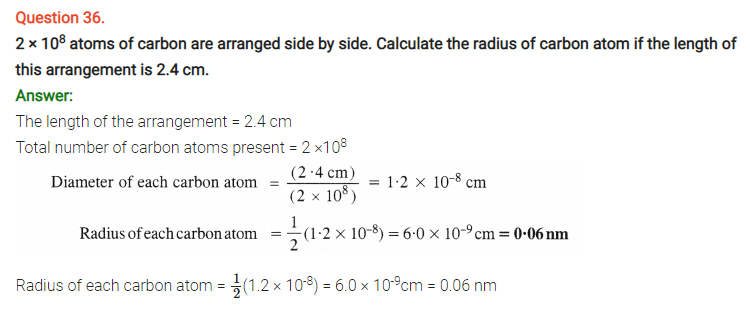
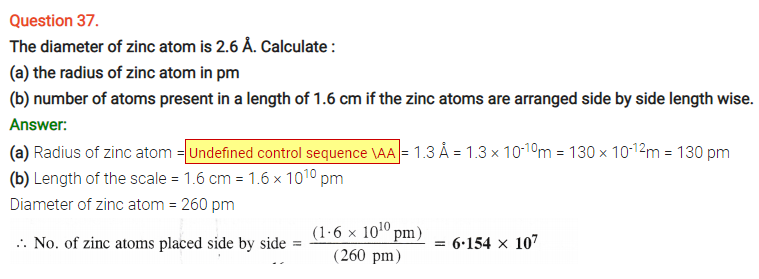
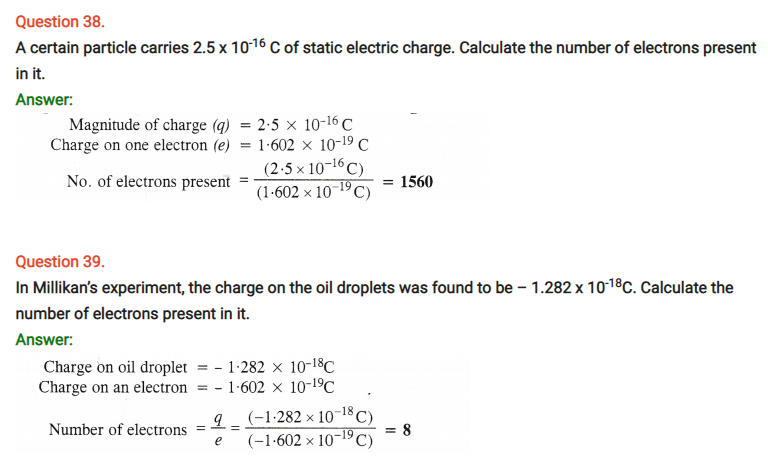


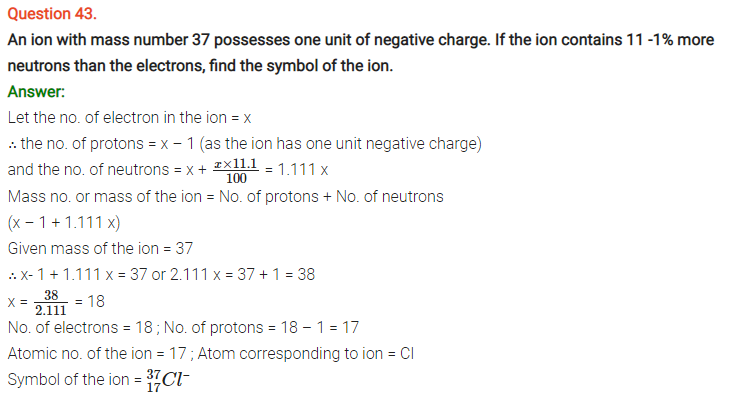


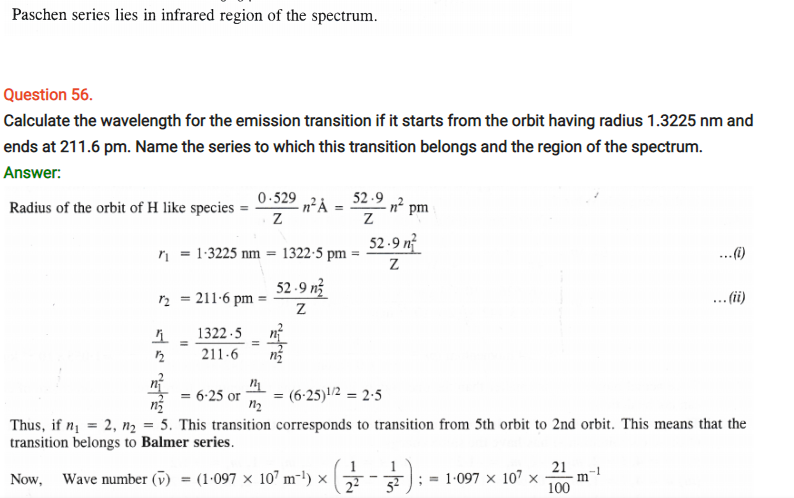
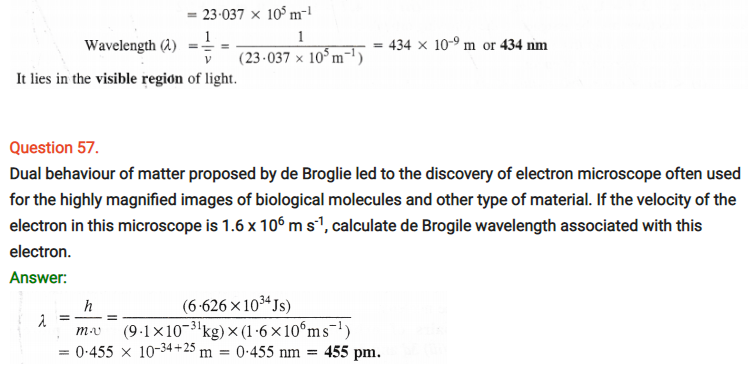

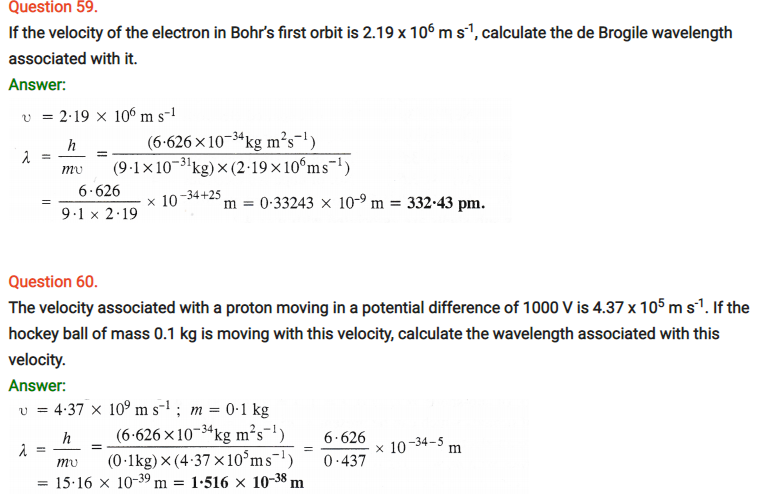
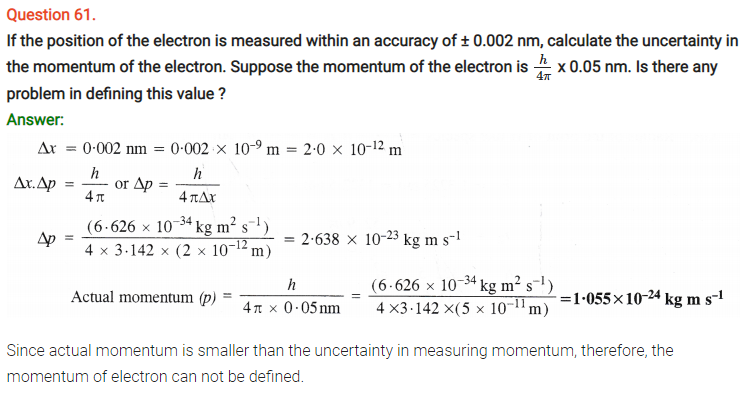

Question 63.
The bromine atom possesses 35 electrons. It contains 6 electrons in 2p orbital, 6 electrons in 3p orbital and 5 electrons in 4p orbital. Which of these electrons experiences lowest effective nuclear charge ?
Answer:
4p electron experiences lowest effective nuclear charge because of the maximum magnitude of screening or shielding effect. It is farthest from the nucleus.
Question 64.
Among the following pairs of orbitals, which orbital will experience more effective nuclear charge (i) 2s and 3s (ii) 4d and 4f (iii) 3d and 3p ?
Answer:
Please note that greater the penetration of the electron present in a particular orbital towards the nucleus, more will be the magnitude of the effective nuclear charge. Based upon this,
(i) 2s electron will experience more effective nuclear charge.
(ii) 4d electron will experience more effective nuclear charge.
(iii) 3p electron will experience more effective nuclear charge.
Question 65.
The unpaired electrons in A1 and Si are present in the 3p orbital. Which electrons will experience more effective nuclear charge from the nucleus?
Answer:
Configuration of the two elements are :
A1 (Z = 13) : [Ne]103s23p1 ; Si (Z = 14) : [Ne] 103s23p2
The unpaired electrons in silicon (Si) will experience more effective nuclear charge because the atomic number of the element Si is more than that of A1.
Question 66.
Indicate the number of unpaired electrons in :
(a) P (b) Si (c) Cr (d) Fe and (e) Kr.
Answer:
(a) P (z=15) : [Ne]103s23p3 ; No. of unpaired electrons = 3
(b) Si (z=14) : [Ne]103s23p2 ; No. of unpaired electrons = 2
(c) Cr (z=24): [Ar]184s13d5 ; No. of unpaired electrons = 6
(d) Fe (z=26): [Ar]184s23d6 ; No. of unpaired electrons = 4
(e) Kr (z=36) : [Ar]184s23d104p6 ; No. of unpaired electrons = Nil.
Question 67.
(a) How many sub-shells are associated with n = 4 ?
(b) How many electrons will be present in the sub-shells having ms value of -1/2 for n = 4 ?
Answer:
(a) For n = 4 ; No. of sub-shells = (l = 0, l = 1, l = 2, l = 3) = 4.
(b) Total number of orbitals which can be present = n2 = 42 = 16.
Each orbital can have an electron with ms = – 1/2 -‘. Total no. of electrons with m, = – 1/2 is 16.

Related link you must like:-
Study material for Competition Exam
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]