www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Engineering post, Career Guidelines , English Speaking Trick , How to crack interview and lots more. Chemistry Solution Notes
Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions:
| Section Name | Topic Name |
| 2 | Solutions |
| 2.1 | Types of Solutions |
| 2.2 | Expressing Concentration of Solutions |
| 2.3 | Solubility |
| 2.4 | Vapour Pressure of Liquid Solutions |
| 2.5 | Ideal and Non-ideal Solutions |
| 2.6 | Colligative Properties and Determination of Molar Mass |
| 2.7 | Abnormal Molar Masses |
Introduction
During summer after returning home from outside it feels refreshing to drink a glass lemon water. What is it actually?? It is nothing just a mixture of salt, sugar, lemon juice in water. This a rehydrating solution. Solution can be defined as a liquid mixture with minor component (salt, sugar, lemon juice) uniformly distributed within the major component (water).
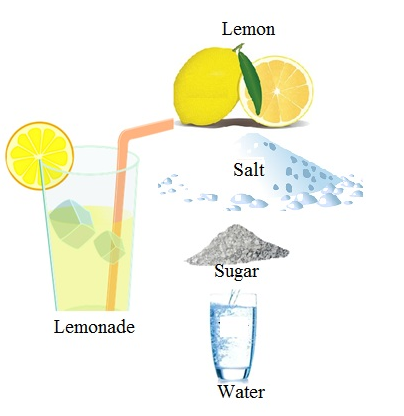
The component of a solution present in the huge quantity is referred to as solvent which determines the physical state of the solution whereas the components of a solution present in the lesser amount is referred to as solute.
In the case of lemonade Water is the solvent whereas salt/sugar are the solute.
Types of solutions
Solutions can be broadly categorized into two types:
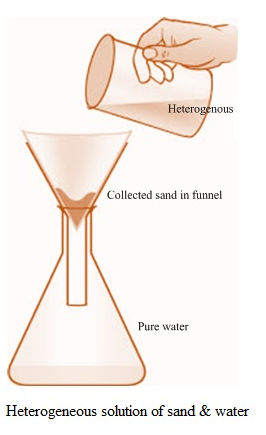
Homogeneous solutions: Solutions with uniform composition and properties throughout the solution are known as homogenous solution. E.g. solution of salt or sugar in water, cough syrup, cup of coffee, Mouthwash, perfume are homogeneous mixture of chemicals and dyes, etc.


| Type of solution | Solute | Solvent | Example |
| Gaseous | Gas | Gas | Oxygen and nitrogen mixture |
| Liquid | Gas | Chloroform with nitrogen gas | |
| Solid | Gas | Camphor in nitrogen gas | |
| Liquid | Gas | Liquid | Oxygen in water |
| Liquid | Liquid | Ethanol in water | |
| Solid | Liquid | Glucose in water | |
| Solid | Gas | Solid | Hydrogen solution in palladium |
| Liquid | Solid | Amalgam of mercury with sodium | |
| Solid | Solid | Dissolved copper in gold |
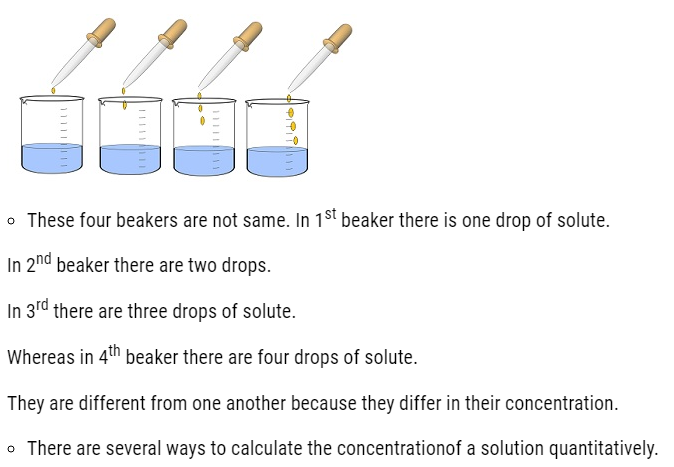
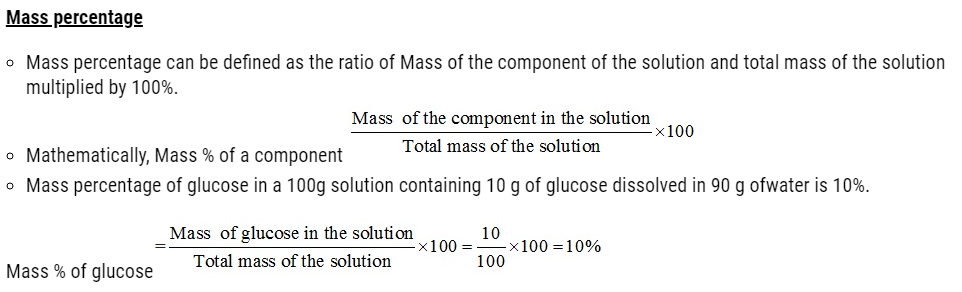
Concentration of solution
- Concentration of a solution is the measure of the composition of a solution.
- A solution with relatively very large quantity of solute is called concentrated solution.
- A solution with relatively very small quantity of solute is called a dilute solution.

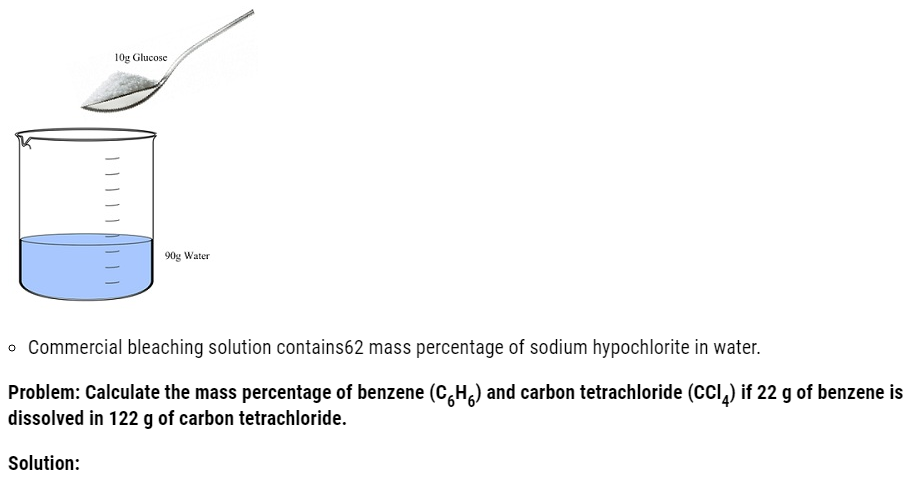
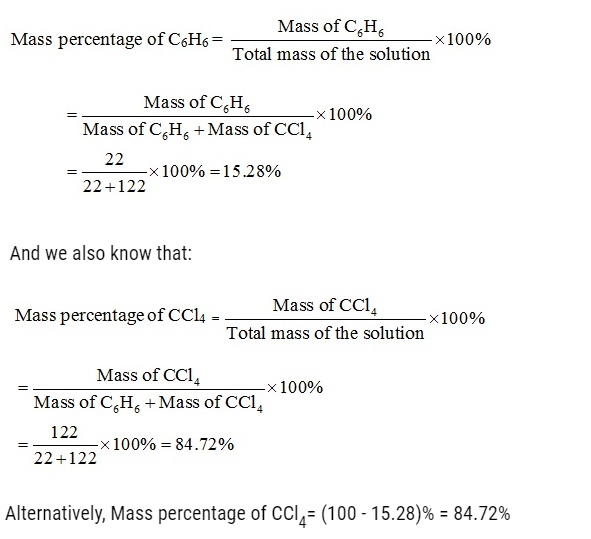
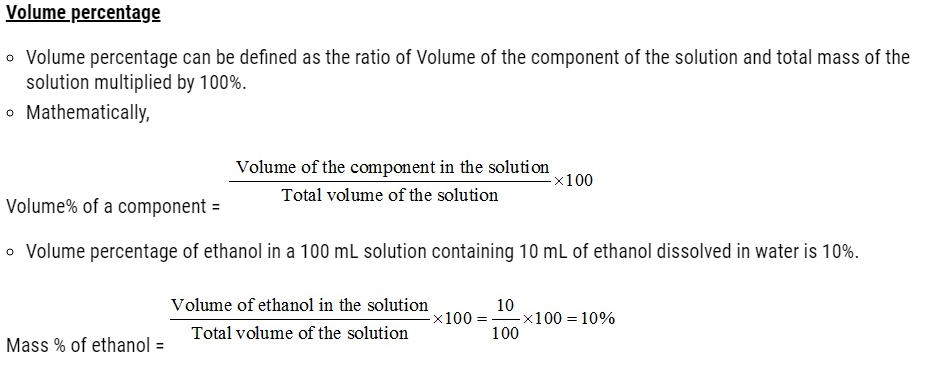

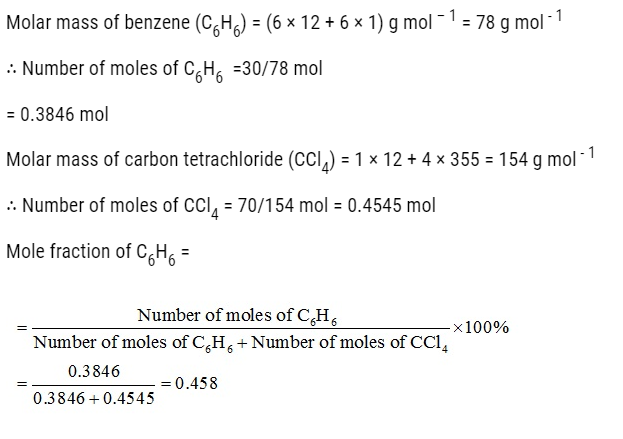
Problem:
Ami took a 20 cm3 mixture of CO, CH4 and He gases and exploded it by an electric discharge at room temperature with excess of oxygen. The volume initially contracted to 13.0 cm3. Then it further contracted to 14.0 cm3 when the residual gas is treated with KOH solution. Find out the consumption of gaseous mixture in terms of volume percentage.
Solution:
Take V1= Partial volumes of CO
V2= Partial volumes of CH4
V3 = Partial volumes of He
V1+V2+V3 = 20.0 cm3
The equation for combustion is as follows:
CO + ½ O2 –> CO2
CH4 + 2O2 –> CO2 + 2H2O
V1 volume of CO = ½ V1 volume of O2 = V1 volume of CO2
V2 volume of CH4 = 2V2 volume O2 = V2 volume of CO2
V1/2 + 2V2 = 13.0 cm3
Treating of residual gases with KOH solution would cause the absorption of CO2
V1+ V2 = 14.0 cm3
V1 = 10 cm3
V2 = 4.0 cm3
V3 = 6.0 cm3
Volume % of CO = 10/20 x 100 = 50
Volume % of CH4 = 4/20 X 100 = 20
Volume % of He = 100 – (50 + 20) = 30










- This is because it is property of solution that polar solutes dissolve in polar solvents like Water, ethanol, formaldehyde and chloroform whereas non polar solutes in nonpolar solvents like pyridine, toluene, and hexane. This phenomenon can be easily defined as like dissolves like.
- The phenomenon of increase in the concentration of solution due to dissolving of solid solute to the solvent is known as dissolution.
- The phenomenon of collision of solute particles in a solution resulting in the separation of the solute particles from the solution is known as crystallization.
- A phase when number of solute particles going into solution is equal to the solute particles separating out of the solution is known as equilibrium. The concentration of solute remains constant at this stage.
- A solution in which no more solute can be dissolved into the solvent at the same temperature and pressure is known as saturated solution.
- Whereas a solution in which more solute can be dissolved in the solvent at the same temperature and pressure is known as unsaturated solution.
- According to Le Chateliers Principle in a saturated solution, if the dissolution process is endothermic (Δsol H > 0), the solubility should increase with rise in temperature but if the dissolution process is exothermic (Δsol H > 0) the solubility should decrease.
- Pressure does not have crucial effect on solubility of solids in liquids because they remain unaffected to pressure due to the high incompressibility of solids and liquids.
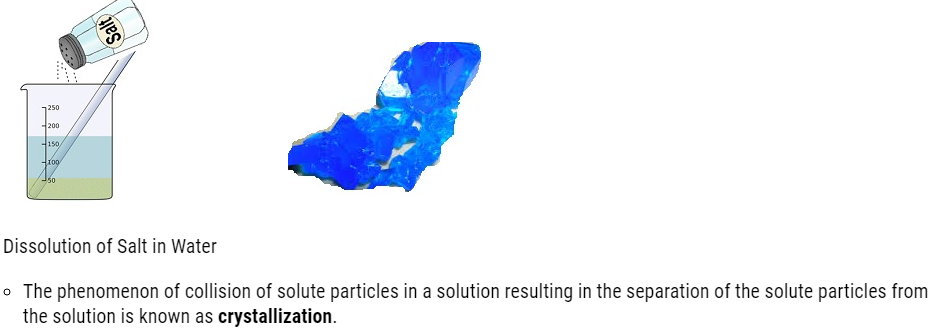
Crystal of copper Sulfate obtained by the crystallization of Copper Sulfate solution
- A phase when number of solute particles going into solution is equal to the solute particles separating out of the solution is known as equilibrium. The concentration of solute remains constant at this stage of dynamic equilibrium.
- Solubility of gas in liquid is highly affected by temperature and pressure.
- Increase in temperature reduces the solubility of gases in liquids because on dissolving gas molecules in liquid the process of dissolution is similar to condensation leading to the evolution of heat. Therefore, dissolution is an exothermic process, due to which the solubility decreases with increase of temperature.
- Increase in pressure by compressing the gas to a smaller volume increase the number of
gaseous particles per unit volume over the solution and also the rate at which the gaseous particles are striking the surface of solution to enter it leading to the increase in the solubility of gases.
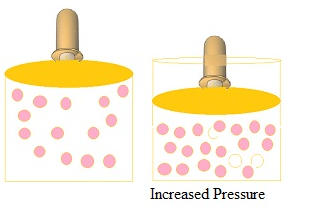
Henry’s law
- Henry’s Law establishes aquantitative relation betweenpressure and solubility of a gasin a solvent.
- This law is for gas-liquid solution.
- According to the law at a constant temperature, thesolubility of a gas in a liquid is directly proportional to thepressure of the gas.
- It can also be stated as – The partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution.
- Mathematically,
p = KH x where KH = Henry’s law constant.
- At same temperature different gasses have different KH
- It is a function of the nature of the gas. At a given pressure increasing value of KH implies lower solubility of the gas in the liquid.
- Value of KH increases with the increase in temperature therefore solubility of gases increases with decreasing temperature. Due to this reason cold water is more sustainable for aquatic life than warm water.
Problem: H2S, a toxic gas with rotten egg like smell, is used for the qualitative analysis. If the solubility of H2S in water at STP is 0.195 m, calculate Henry’s law constant.
Solution:
It is given that the solubility of H2S in water at STP is 0.195 m, i.e., 0.195 mol of H2S is dissolved in 1000 g of water.
Moles of water = 1000g / 18g mol-1 = 55.56 mol
∴Mole fraction of H2S, x = Moles of H2S / Moles of H2S+Moles of water 0.195 / (0.195+55.56)= 0.0035
At STP, pressure (p) = 0.987 bar
According to Henry’s law:
p = KH x ⇒ KH = p / x
= 0.0987 / 0.0035 bar
= 282 bar


Raoult’s law
- Raoult’s law establishes a quantitative relationship between the partial vapour pressure and mole fraction of a solution.
- This law is only for liquid-liquid solution.
- The law states that for a solution of volatile liquids, the partial vapour pressure (p) of each component in the solution is directly proportional to its mole fraction (x).
- Mathematically, p ∝ x
Or p = p 0x
Where p0 is the vapour pressure of pure component at the same temperature.
- Thus, for component 1, p1 = p1 0 x1 and for component 2 p2 = p20 x
- Let the solvent be represented by 1 and solute be represented by 2. At the point when the solute is non-volatile, just the solvent molecules are present in vapour phase and add to vapour pressure.
Let p1 = vapour pressure of the solvent,
x1 = mole fraction,
pi0 = vapour pressure in the pure state.
According to Raoult’s law
p1 ∝ x1
And p1 = p1 0 x1



p2= Partial vapour pressures of component 2
x1 = Mole fractions of component 1.
x2 = Mole fractions of component 2
- Dalton’s law of partial pressures states that the total pressure (ptotal) of the solution is the sum of the partial pressures of the components of the solution.
- Mathematically, P total = P1 + P2
- Substituting the values of p1 and p2 we get

P1 = y1 Ptotal
P2 = y2 Ptotal
Vapour pressure of solution of solids in liquids
- Solution of salt, sugar or glucose dissolved in water is solutions of solids in liquids.
- Similarly iodine and sulphur dissolved in carbon disulphide are also solutions of solids in liquids
- Addition of anon-volatile solute to a solvent togive a solution results in the exertion of the vapourpressure of the solution uniquely from the solvent.
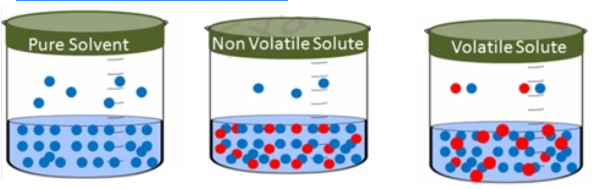
- This vapour pressure is lower than the vapour pressure of the pure solvent at a given temperature.
- The surface of the solution is occupied by both solute andsolvent molecules which results in the reduction of the surface for the solvent molecules. As a result the apparent number ofsolvent molecules escaping from the surface is reduced. This in turn reduces the vapour pressure.
- The reduction of vapour pressure of solvent depends on thequantity of non-volatile solute present in the solution not on its nature.


Positive deviation from Raoult’s Law
- The solute- solute and solvent-solvent interaction are stronger than solute-solvent interaction.
- Let pA = Partial vapour pressures of component A
PB = Partial vapour pressures of component B
Hmix = Change in enthalpy of mixing.
Vmix =Change in volume of mixing.
- pA > pAoxA
- pB > pBoxB
- ΔHmix > 0
- ΔVmix > 0
- Total vapor pressure is more than as expected from Raoult’s law.
- Solution of ethanol and acetonebehaves in the same manner. Molecules of pure ethanol are hydrogen bonded. The molecules of acetone get in between the molecules of ethanol on addition of acetone and break some of the hydrogen bonds between them.

Negative deviation from Raoult’s Law
- The solute-solute and solvent –solvent interactions are weaker than solute-solvent interaction.
- Let pA = Partial vapour pressures of component A
PB = Partial vapour pressures of component B
Hmix = Change in enthalpy of mixing.
Vmix =Change in volume of mixing.
- pA< pAoxA
- pB < pBoxB
- ΔHmix < 0
- ΔVmix < 0
- Total vapor pressure is less than as expected from Raoult’s law.
- Solution of phenol and aniline. It has strong intermolecular hydrogen bonding between phenolicproton and lone pair of nitrogen atom of aniline than the intermolecular hydrogen bonding between similar molecules.
- A solution of chloroform and acetone shows negative deviation from Raoult’s law due to the formation of hydrogen bond with acetone molecule by chloroform molecule. This decreases the chances of molecules of component to escape leading to the decrease in vapour pressure. And hence the solution exhibits negative deviation from Raoult’s law

Azeotropes
- Azeotropes are binary mixturespossessing the same synthesis in fluid, vapor stage and boiling stage at a steady temperature.
- The components of azeotropic mixtures are separated by fractional distillation.
- Azeotropes can be broadly categorized into two types-
- Minimum boiling azeotrope
The solutions showing positive deviation from Raoult’s law. For instance, solution of ethanol and water on fractional distillation results in a solution containing approximately 95% by volume of ethanol.
- Maximum boiling azeotrope.
The solutions showing negative deviation from Raoult’s law. For instance, solution of nitric acid and water has the approximate arrangement, 68% nitric corrosive and 32% water by mass, with a breaking point of 393.5 K.
Properties of solution affected by vapour pressure
- Vapour pressure of solution decreases on addition of a non-volatile solute to a volatile solvent.
- Properties of solutions affected by the decrease of vapour pressure includes-
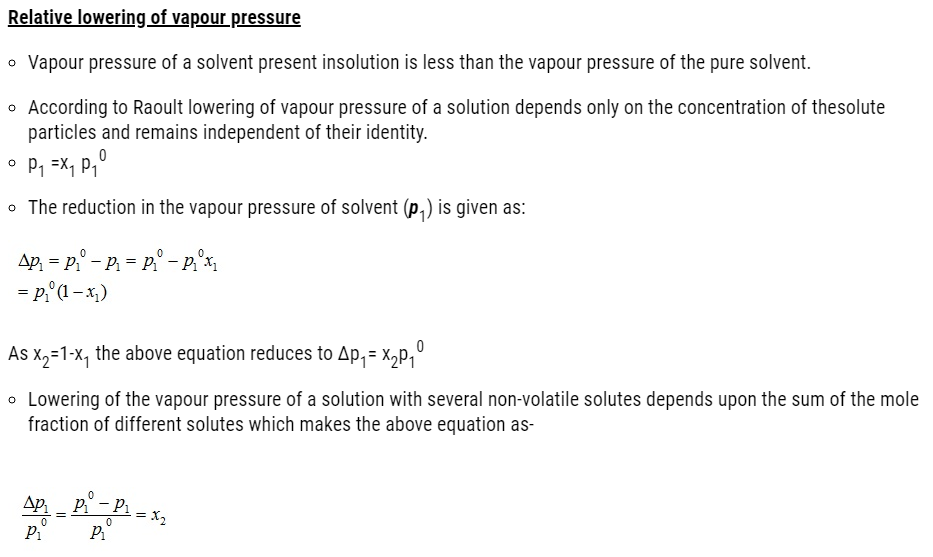
- Relative lowering of vapour pressure of the solvent
- Depression of freezing point of the solvent
- Elevation of boiling point of the solvent and
- Osmotic pressure of the solution.
- These properties of solution depend on the number of solute particles present in the solution regardless of their nature relative to the total number of particles present in the solution. These properties are termed as colligative properties derived from a Latin word with co meaning together ligare meaning to bind.
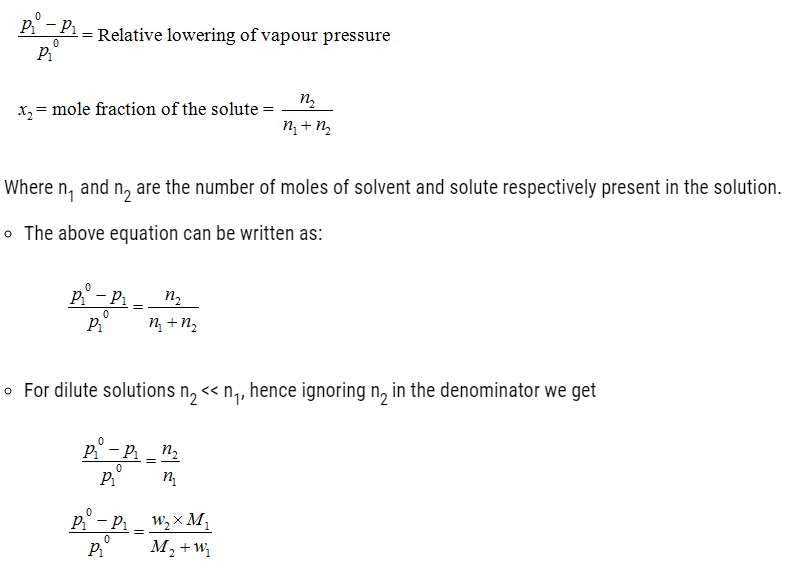
w1 and M1 = Masses and molar masses of solvent.
w2 and M2 = Masses and molar masses of solute.
Elevation of boiling point
- Increase in temperature increases the vapour pressure of a
- A liquid boils at atemperature where the vapour pressure is equal to the atmospheric
- Vapourpressure of the solvent decreases in the presence of non-volatile solute.
- The boilingpoint of a solution is always higher than that ofthe boiling point of the pure solvent in which thesolution is prepared as.
- The elevation ofboiling point also depends on the number ofsolute molecules rather than their nature.
- The increase in the boiling point = ΔTb = Tb – Tb0. This is known as elevation of boiling point.
Tb0 = boiling point of pure solvent
Tb0 = boiling point of solution
- In dilute solutions the elevation of boiling point Tb is directly proportional to the molal concentration of the solute in a solution. Mathematically,
ΔTb ∝ m
ΔTb = kb m
m = Molality = number of moles of solute dissolved in 1 kg of solvent
Kb = constant of proportionality. It is also known as Boiling Point Elevation Constant or Molal Elevation Constant (Ebullioscopic Constant). The unit of Kb is K kg mol-1.
- Let w2 and M2 = Masses and molar masses of solute
w1= Mass of solvent
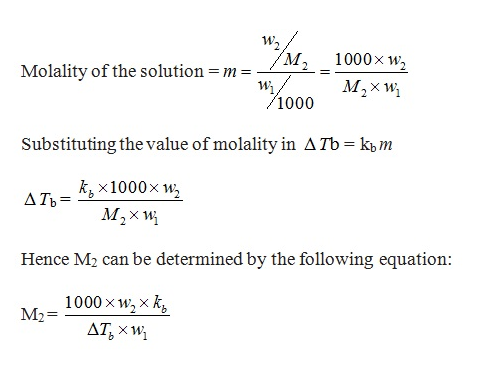
Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose is to be added to 500 g of water such that it boils at 100°C.Molal elevation constant for water is 0.52 K kg mol-1.
Sol. Elevation of boiling point ΔTb= (100 + 273) – (99.63 + 273) = 0.37 K
Mass of water, wl = 500 g
Molar mass of sucrose (C12H22O11),
M2= 11 × 12 + 22 × 1 + 11 × 16 = 342 g mol – 1
Molal elevation constant, Kb= 0.52 K kg mol – 1
We know that:

Depression of freezing point
- Freezing point of a substance is the temperature at which the vapour pressure
of the substance in its liquid phase is equal to the vapour pressure in the solid phase. - If the vapour pressure of the solution is equal to the vapour pressure of the pure solid solvent then the solution freezes.
- According to Raoult’s law, addition of non-volatile solute decreases the vapour pressure of the solvent and would be equal to that of solid solvent at lower This decreases the freezing point of thesolvent.
- The decrease in the freezing point = ΔTf = Tf – Tf0. This is known as depression in freezing point.
Tf0 = freezing point of pure solvent
Tf0 = freezing point when non-volatile solute is dissolved
- In a dilute solution the depression of freezing point Tf is directly proportional to molality of the solution. Mathematically,
ΔTf ∝m
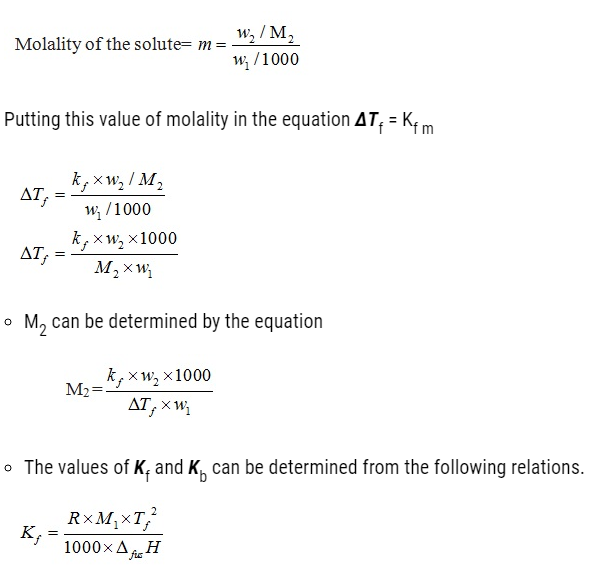
ΔTf = Kf m
Kf = Proportionality constant depending upon the nature of the solvent. This is known as Freezing Point Depression Constant or Molal Depression Constant or Cryoscopic Constant. The unit of Kf is K kg mol-1.
- Let w2 and M2 = Masses and molar masses of solute
w1= Mass of solvent


Osmosis and Osmotic pressure
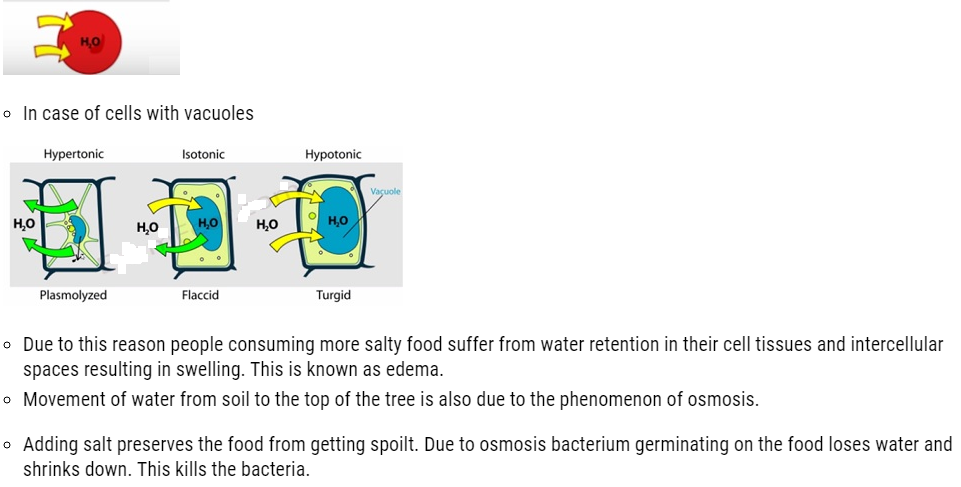
- We have often observed that raw mangoes shrink when kept in salt water (brine solution), flowers remain fresh for longer time when kept in water.
- This is due to the fact that small solvent molecules such as water are capable of passing through the pores present in every cell. These pores allows small solvents like water to pass through but hinders the passing of larger molecules. This membrane possessing such pore is known as semipermeable membranes.
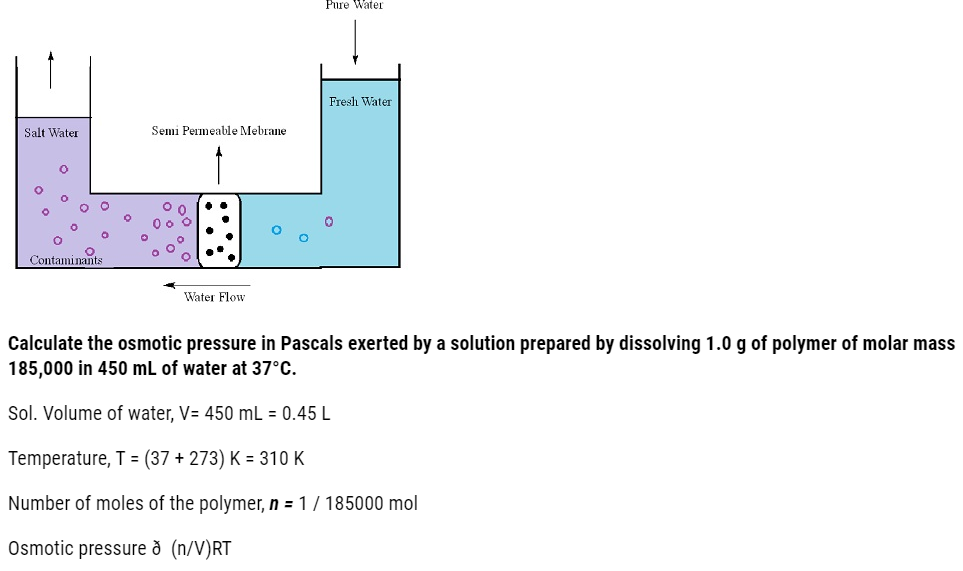
- The process of flow of solvents from the pure solvent to the solution through a semipermeable membrane is termed as osmosis.
- This flow of solvents to the solution continues until the stage of equilibrium is reached.
- Application of extra pressure on the solution can terminate the flow of solvents from the pure solvent to the solution. This pressure is called osmotic pressure of the solution. It is a colligative property that depends upon the concentration of the solution and is basically meant to stop the passage of solvent molecules through a semipermeable membrane into the solution.
- Solvent molecules generally flows from lower concentration to higher concentration. This flow of solvent from dilute solution to the concentrated solution through a semipermeable membrane is due to the phenomenon of osmosis.
= 1/185000 mol X 1/0.45L X 8.314 X 103 Pa L K-1mol-1 X 310K
= 30.98 Pa = 31 Pa
Calculation of Molar Mass from Osmotic Pressure
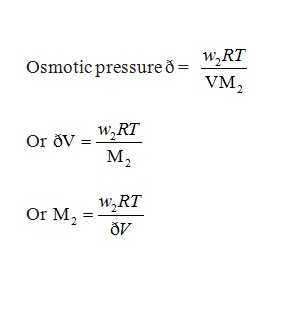
In dilute solutions the osmotic pressure is proportional to the molarity, C of the solution at a given temperature T. Mathematically,
ð = C R T
R= gas constant
ð = osmotic pressure = (n2/V) RT
V = Volume of a solution in litres
n2 = Moles of solute.
w2 = Grams of solute
M2 = Molar mass
Now, n2 = w2/M2



Use of Reverse osmosis in water purification
Reverse osmosis is extensively used in the desalination of sea water.
A porous film of cellulose acetate is used as a membrane which is permeable to water but impermeable to impurities and ions is placed over a support.
Pressure larger than the osmotic pressure is applied on the sea water solution which allows the passing out of the pure water holding back the bulk of contaminants through the membrane made of polymer.
The measure of pressure required relies upon the salt grouping of the food water. Greater concentration of feed water requires more pressure to defeat the osmotic pressure.
As the water enters the RO membrane under great pressure the water molecules pass through the semi-permeable membrane.
The membrane is impermeable to salts and other contaminants and hence is not allowed to pass through it.
These leftover contaminated water is then discharged through the reject stream and finally drained out or is fed back into the feed water supply. This water will again undergo same procedure and will be purified in some way.

Abnormal molar mass
- Some molecules of solvents with low dielectric constant like molecules of ethanoic acid dimerize into benzene due to the presence of hydrogen bond.
- Association of all the molecules of ethanoic acid into benzene means Tb or Tf for ethanoic acid will be half of the normal value.
- The molar mass calculated on the basis of this ΔTb or ΔTf will be twice the expected molar mass.
- This value of molar mass is either lower or higher compared to expected or normal value and is known as abnormal molar mass.
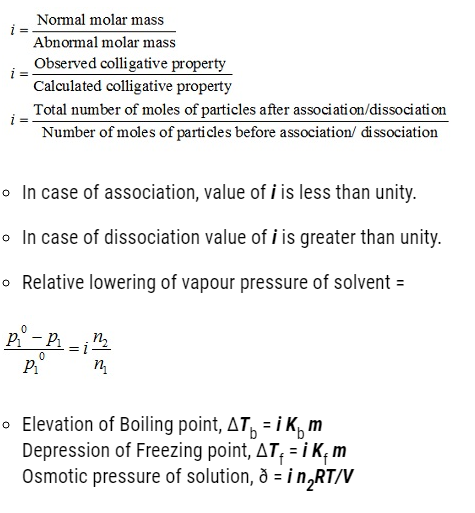
- In 1880 van’t Hoff introduced a factor i and was named as the van’t Hoff factor.
- The van’t Hoff factor account for the degree of dissociation or association.
- The van’t Hoff factor i can be defined as the ratio of Normal molar mass to the abnormal molar mass. Mathematically,
Chapter 2: Solution
Now Let’s start the chapter 2 chemistry [Solution] important topic and question for students of class 12th.
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]






