www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Engineering post, Career Guidelines, English Speaking Trick, How to crack an interview, and lots more. ( Redox Reactions Class 11th Chemistry Notes and NCERT Solution )
NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reactions

Chapter 8 Redox Reactions:
| Section Name | Topic Name |
| 8 | Redox Reactions |
| 8.1 | Classical Idea of Redox Reactions-Oxidation and Reduction Reactions |
| 8.2 | Redox Reactions in Terms of Electron Transfer Reactions |
| 8.3 | Oxidation Number |
| 8.4 | Redox Reactions and Electrode Processes |
Introduction
The reaction in which oxidation and reduction both occur simultaneously are called Redox reactions. Let us understand about oxidation and reduction. We have different concepts to understand oxidation and reduction.
Oxidation and Reduction
- In terms of Classical concept
Oxidation: Oxidation is defined as “addition of oxygen or any electronegative element and removal of hydrogen or any electropositive element”.

Please note the substance that undergo oxidation itself and reduce other is called reducing agent whereas the substance that undergo reduction itself and oxidize other is called oxidizing agent.
For example:- CuO + H2 –> Cu + H2O
Copper Oxide Hydrogen Copper Water
In reaction, Cu is undergoing reduction and oxidizing hydrogen. Therefore, Cu is oxidizing agent and H is reducing agent.
“As in this reaction both oxidation and reduction occur simultaneously, therefore reaction is called redox reaction.”
- In terms of Electron transfer concept
Oxidation: Is defined as loss of electrons that is:
M-electron –> M+
Metal Metal ion
Reduction: Is defined as gain of electrons that is given below.
N + electron –> N–
Non metal non metal ion
Let us explain it more with the help of example:
Let’s consider equation: H2 + O2 — > H2O
We can see in this example that H atom as going from neutral to a positive state in water, the O atom goes from zero state to dinegative state in water. There is an electron transfer from H to O and consequently H2 is oxidized and O2 is reduced .The charge transfer is only partial or we can say that it is electron shift rather than calling it as complete loss of electron by H and gain by O.
Another example of Redox reaction in terms of electron transfer:
Mg + H2 –> MgH2
Magnesium Hydrogen MagnesiumHydride
In this Magnesium is losing electrons, that is undergoing oxidation and hydrogen is gaining electrons therefore, it is undergoing reduction. Mg is Reducing agent in it and H is Oxidizing agent in it.
- In terms of oxidation number
Oxidation number: It is defined as “residual charge that is present on atom when the atom is in combined state with other atoms”.
Rules to assign and calculate oxidation number
- The oxidation number of atoms in their elemental state is taken as zero.
- The oxidation number of mono-atomic atoms like Na+ etc is taken as 1.
- The oxidation number of Hydrogen is +1 when present with non metals and -1 when present with metals.
- The oxidation number of oxygen is -2 in most of the compounds but in peroxides it is -1.
- The metals always have oxidation number in positive and non metal in negative when present together in ionic compounds.
- In compounds that have two atoms with different electro negativities, the oxidation number of more electronegative is taken as –ve and for less electronegative it is taken as positive. For example: In OF2 the oxidation number of oxygen will be in positive and oxidation of fluorine will be in negative.
- In neutral compounds, the sum of all oxidation numbers is equal to zero.
- In complex ions, the sum of oxidation states of all the atoms is equal to the charge present on the complex.
Oxidation: It is defined as increase in oxidation number of an element.
Mg + H2 –> Mg2+ H2
Magnesium Hydrogen MagnesiumHydride
In this, Magnesium oxidation number is increased from 0 to +2.Therefore, oxidation occurs.
Reduction: It is defined as decrease in oxidation number of an element.
For Example:-
Fe3+ + e– –> Fe2+
Ferric Ion Ferrous Ion
In this oxidation number of iron is reduced .Therefore, reduction occurs.
Redox reaction in terms of oxidation number
In this, Zinc is undergoing oxidation and act as reducing agent whereas Hydrogen is undergoing reduction and act as an oxidizing agent.
Zn0 + H+Cl– –> Zn2+ + 0H2
Zinc HydrogenChloride Zinc Chloride Hydrogen Gas
Balancing of Redox reactions
There are two ways of balancing Redox reactions:
- Oxidation number method
- Half equation method
Oxidation method: The steps to be followed-
- Write the skeletal equation of reactants and products.
- Indicate the oxidation number of all the elements involved in the reaction.
- Calculate the increase or decrease in oxidation number per atom. Also, identify the oxidizing and reducing agents.
- Multiply the formula of oxidizing agent and reducing agent by suitable integers, so as to equalize the total increase or decrease in oxidation number as calculated in step c.
- Balance all atoms other than H and O.
- Finally balance H and O atoms by adding water molecules using hit and trial method.
- In case of Ionic reactions:
- For acidic medium
- First balance O atoms by adding water molecules to the deficient side.
- Balance H+ ions to the side deficient in H atoms.
- For Basic medium
- First balance oxygen atom by adding water molecules to the deficient side.
- Then to balance hydrogen, add water molecules equal to the number of deficiency of H atoms.
- Also add equal number of OH– ions to opposite side of the equation.
Example: Permagnate ion reacts with bromide ion in basic medium to give manganese dioxide and Bromate ion .
Step1: the skeletal ionic equation is :
MnO4– (aq) +Br– (aq) —> MnO2 +BrO3–
Step 2: assign oxidation numbers for Mn and Br

Half reaction method or Ion electron method
- Write the skeletal equation and indicate the oxidation number of all the elements which occur in skeletal equation
- Find out the species that are oxidized and reduced.
- Split the skeletal equation into two half reactions: oxidation half reaction and reduction half reaction
- Balance the two-half equation separately by rules described below:
- In each half reaction first balance the atoms of element that has undergone a change in oxidation number.
- Add electrons to whatever side is necessary to make up the difference in oxidation number in each half reaction.
- Balance the charge by adding H+ ions, if the reaction occurs in acidic medium .For basic medium, add OH– ions if the reaction occurs in basic medium.
- Balance oxygen atoms by adding required number of water molecules to the side deficient in oxygen atoms
- In the acidic medium, H atoms are balanced by adding H + ions to the side deficient in H atoms.
- However, in the basic medium H atoms are balanced by adding water molecules equal to number to H atoms deficient.
- Add equal number of OH– ions to opposite side of equation.
- The two half reactions are then multiplied by suitable integers .so that the total number of electrons gained in half reaction becomes equal to total number of electrons lost in another half reaction.
- Then the two half reactions are added up.
- To verify the balancing, check whether the total charge on either is equal or not.
Example: Let us consider the skeletal equation:
Fe2+ + Cr2O72- –> Fe3+ +Cr3+
Step 1: Separate the equation in to two halves:
Oxidation half reaction: Fe2 –>Fe3+
Reduction half reaction: Cr2O72- –> Cr3+
Step 2: Balance the atoms other than hydrogen and oxygen in each half reaction individually. Here the oxidation half reaction is already balanced with respect to Fe atoms .For the reduction half reaction, we multiply the Cr3+ by 2 to balance Cr atoms.
Step 3: For reactions occurring in acidic medium, add water molecules to balance oxygen atoms and hydrogen ions are balanced by adding H atoms. Thus, we get:
Cr2O72- + 14 H+ + 6e– –> 2 Cr3+ + 7H2O
Step 4: Add electrons to one side of the half reaction to balance the charges .if needed make the number of electrons equal in two half reactions by multiplying one or both half reaction by suitable coefficient.
The oxidation half reaction is thus written again to balance the charge .Now in the reduction half reaction there are 12 positive charges on the left hand side and only 6 positive charge on right hand side .Therefore, we add six electrons to left hand side .
Cr2O72- + 14 H+ + 6e– –> 2 Cr3+ + 7H2O
To equalize the number of electrons in both reactions, we multiply oxidation half reaction by 6 and write as:
6Fe2+ –> 6Fe3+ +6e–
Step 5: We add the two half reactions to achieve the overall reaction and cancel the electrons on each side .This give us net ionic equation:
6Fe2+ + Cr2O72- + 14 H+ –> 2Cr3++6Fe3+ +7H2O
Step6: Verify that the equation contains the same type and number of atoms and the same charges on both sides of the equation. This last check reveals that the equation is fully balanced with respect to number atoms and the charges.
Types of Redox reactions
- Combination reaction: When two or more reactants react to form single product.
i.e a + b –> ab (in this single product is formed).
For the reaction to be Redox, both the elements should be in elemental form.
For example: All combustion reactions are Redox reactions.
C0 + O2 –> CO2
Carbon Oxygen Carbondioxide


There are two types of Redox reactions
- Direct Redox reaction
- Indirect Redox reaction
Direct Redox reaction: In which oxidation and reduction both occur in same beaker. In this electron so produced does not travel to large distance.
For example: A beaker containing Zinc rod dipped in Copper sulphate solution in this the following reaction occur:
Zn + CuSO4 –> ZnSO4 + Cu
Zinc CopperSulphate Zinc Sulphate Copper
In this zinc being more reactive displaces copper from copper sulphate and forms zinc sulphate and copper.
Indirect Redox reactions: In it oxidation and reduction occur in different beakers. The electron so produced has to travel a certain distance that leads to generation of current.
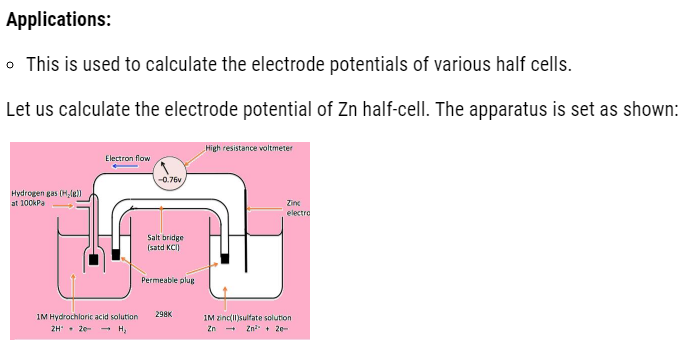
Example: Daniel cell: A cell containing Zn-Cu couple that we are going to study in detail now as given below but before that let us make you familiar with the general term used in redox reaction that is Redox couple.
Redox couple
It is defined as having together the oxidized and reduced forms of a substance taking part in oxidation or reduction half reactions .they are actually conjugate acid base pairs .we can also define it as : an oxidizing and reducing agent which appear on opposite sides of a half equation constitute the redox couple .Like, given below is the cell in which we consider Zn/Cu redox couple .
Electrochemical cell
- Electrochemical cell is the cell in which chemical energy gets converted to electric energy.
- In it indirect redox reactions takes place.
- These reactions are spontaneous that is free energy change for this reaction is negative.
- This cell consists of two half cells.
- In one half cell , there is a aqueous 1molar Zinc sulphate solution with Zinc rod dipped in it.
- In other half cell, there is a 1 molar aqueous solution of Copper sulphate solution with Copper rod dipped in it.

Observations
- With time we see that Zinc rod loses weight, as it has more tendency to loose electrons that is:
Zn -2 electrons –> Zn2+ (Oxidation)
Zinc Zinc Ion
- These electrons released by zinc, travel to another beaker by means of wire. In doing so, they cause deflection in galvanometer and produce current. This current travel in the direction opposite to the flow of electrons.
- These electrons move to another half cell, where copper ions gain these electrons that is reduction occur. As a result, copper metal start depositing on electrode. The reaction that occurs is shown below:
Cu2+ + 2electrons –> Cu(reduction)
Copper ions Copper Metal
Functions of salt bridge
- It connects the circuit internally by connecting the solutions.
- It helps in maintain neutrality.
With passage of time, the left container will have excess positive charge around electrode. Due to which further oxidation stops .Whereas in other beaker negative charge will exceeds, which will start repelling electrons. Therefore, at that time salt bridge comes into action. The oppositely charged electrolyte ions start diffusing into half cells in order to neutralize the excess charge. Hence, the cell keeps on working.
The electrolyte that is selected must fulfill two conditions:
- Size of its cation and anion should be equal.
- Electrolyte in salt bridge should not interact with the main electrolyte of half cells.
The overall reaction that takes place is:
Zn + Cu2+ –> Zn2+ + Cu
Zinc Copper Zinc Ion Copper Metal
Representation of the cell

Electrode potential
Electrode potential is defined as “potential difference set up between electrode and electrolyte of same beaker”.
It is of two types:
- Reduction potential: Tendency of solution to get reduced.
- Oxidation potential: Tendency of electrode to get oxidized.
Factors on which electrode potential depends:
- Concentration of ions in solution.
- Nature of metal and its ions.
Electromotive force
It is the potential difference between two electrodes when no current flows through the circuit.
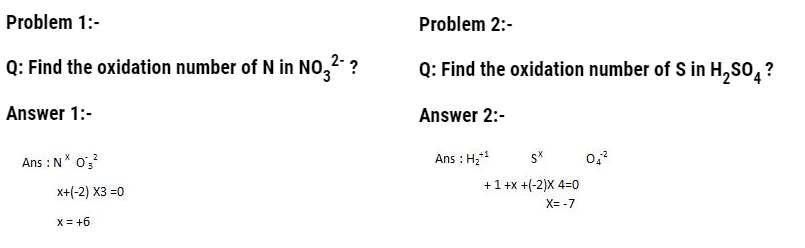
Standard hydrogen electrode and its application
Standard hydrogen electrode is the reference electrode that is used to calculate electrode potential of any electrode. It is also called as SHE or NHE.
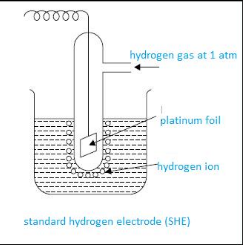
This apparatus consists of beaker having 1 molar HCL solution. In it, a sealed tube having platinum wire is dipped. This platinum wire is further attached to platinum foil. This complete cell is connected to the cell. Then continuously hydrogen gas maintained at 1 atm is bubbled. Platinum foil here acts as a site of reaction.
The Standard electrode potential of SHE is zero volt. This SHE can act as anode or cathode, depending upon the half cell that is attached to it.
If it acts as cathode then following reaction occurs:
2H+ + 2e– –> H2 (Reduction Occurs)
Hydrogen Ion Hydrogen Gas
If it acts as anode then following reaction occur:
2H – 2e– –> 2H+ (Oxidation occurs)
Hydrogen Gas Hydrogen Ions

Electrochemical series
Is the arrangement of elements in order of increasing potential. It is the series has the values starting from –ve to positive. The series is shown below:
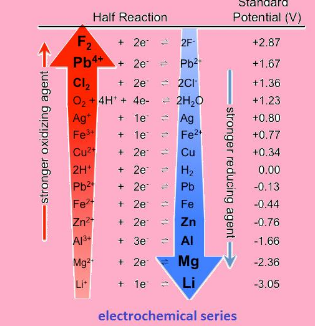
Application:
- The series tells us about the strength of reducing or oxidizing agents
“Lower the value of potential, stronger is the reducing agent “or vice versa.
- The series tells us how to calculate standard electrode potential for cell that is :
E* = (Ec – Ea)
- The series helps us to predict the feasibility of reaction.
- ‘If value of E* is positive , then the reaction is feasible
- If not positive, than the given reaction is not feasible “
- The series helps us to know which metal will evolve hydrogen gas and which will not.
“All the metals with negative reduction potential will evolve hydrogen and others do not.”
Redox reaction as the basis for Titrations
Titration is the process in which the solutions of two reagents are allowed to react with each other.
Procedure:
- In it one solution (known volume) is taken in Burette and the solution is called titrant.
- The other reagent is taken in flask called titration flask and the solution is called as analyte.
- The titration is carried out till both the reagents mix completely.
- The stage at which both the reagent mix completely is called end point.
- The end point is detected by indicator.
The objective of these titrations is to find out the exact amount of an acid (or the base) present in a given solution by reacting it against the solution of standard base (or an acid) .
Introduction
We have studied about so many elements till now .Out of 114 elements, most of them occur in combined state. Only noble gases occur in atomic state otherwise, most of them exist in molecular or combined form.
The combined state is formed by combination of elements that is a chemical bond. Like families, have connections in the same way the attraction of chemical species is called chemical bond.
The reason behind the formation of chemical bond is to obey octet rule .According to this rule, “every element needs to attain stability. For this, the elements either loose or gain electrons to attain 8 electrons in its outermost shell” .But certain violation were seen like in case of hydrogen, the atom has tendency to attain only 2 electrons in valence shell .So, in case of it the rule is violated and is called as Duplet rule.
Other violations are: In certain molecules, the deficient octet is seen. For example in H2 ,whereas in others the central atom has expanded octet that is more than 8 electrons like in SF6.
When two atoms come closer, their outer electrons repel each other and finally they stop at a distance where the released energy is more .As less energy corresponds to maximum stability than at that distance the chemical bond is formed.
Bond formation takes place due to loss of energy and stable state.
Related link you must like:-
Study material for Competition Exam
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]






