www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Engineering post, Career Guidelines , English Speaking Trick , How to crack interview and lots more. ( Haloalkanes and Haloarenes )
- Important Questions Chapter 10 – Haloalkanes and Haloarenes
- NCERT Solutions – INTEXT Questions
- NCERT EXERCISES
NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes:
| Section Name | Topic Name |
| 10 | Haloalkanes and Haloarenes |
| 10.1 | Classification |
| 10.2 | Nomenclature |
| 10.3 | Nature of C–X Bond |
| 10.4 | Methods of Preparation of Haloalkanes |
| 10.5 | Preparation of Haloarenes |
| 10.6 | Physical Properties |
| 10.7 | Chemical Reactions |
Chemistry Haloalkanes and Haloarenes Notes
Introduction
Halo alkanes are the compounds containing halogen atom attached to the sp3 hybridized carbon atom of an alkyl group.
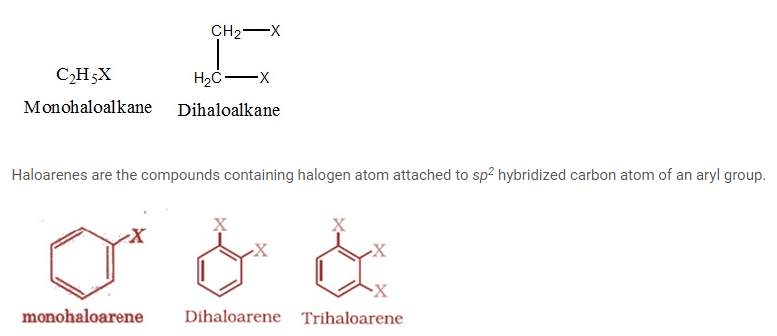
Classification of halo alkanes and halo arenes On the Basis of number of halogen atoms
Depending on number of halogen atoms present in halo alkanes and halo arenes they can be classified as mono (one atom), di (two atoms), or Polyhalogen (tri- (three atoms), tetra- (four atoms), etc.) compounds.
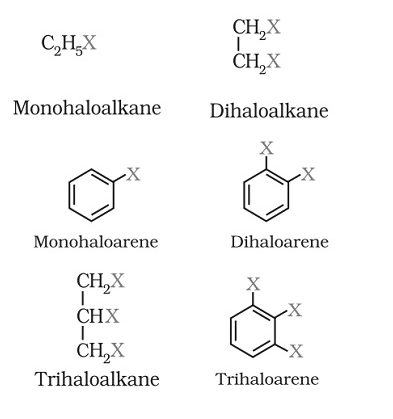
Classification of halo alkanes and halo arenes on the basis of compounds containing sp3C—X Bond
(a) Alkyl halides or halo alkanes (R—X)
They create a homologous series represented by CnH2n+1X and can be further classified as primary, secondary or tertiary.
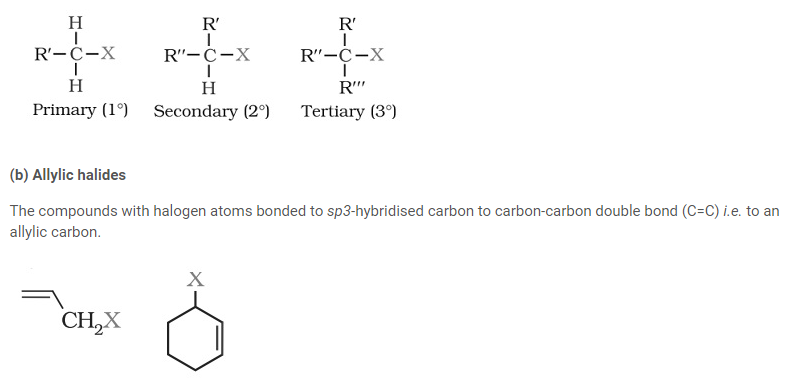
(c) Benzylic halides
The halogen atom of these compounds is bonded to sp3-hybridised carbon atom placed next to an aromatic ring

(b) Aryl halides
The halogen atom of these compounds is bonded to the sp2-hybridised carbon atom of an aromatic ring.
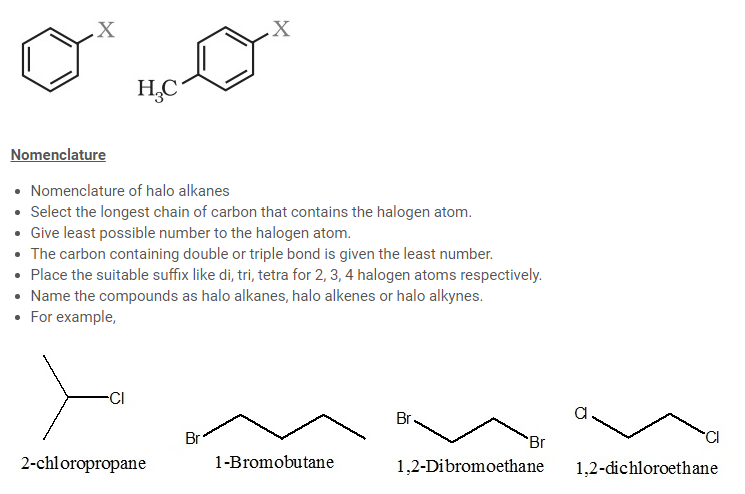
- Nomenclature of halo arenes
- Commonly they are termed aryl halides.
- Numerical prefixes (1,2) ; (1,3) ; (1,4) for [for positions of the compound with respect to halogen atom.
- The halogen atom is given the least number.
- For example,

Problem:
Give the IUPAC names of the following compounds:
(i) CH3CH(Cl)CH(Br)CH3
(ii) CHF2CBrClF
(iii) ClCH2C≡CCH2Br
(iv) (CCl3)3CCl
(v) CH3C(p-ClC6H4)2CH(Br)CH3
(vi) (CH3)3CCH=CClC6H4I-p
Solution:

Problem:
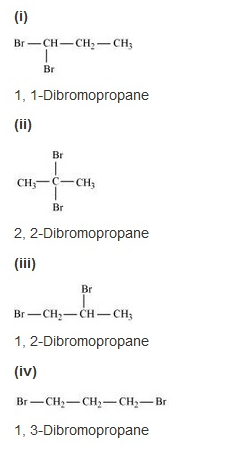
Write structures of different dihalogen derivatives of propane.
Solution:
Problem:
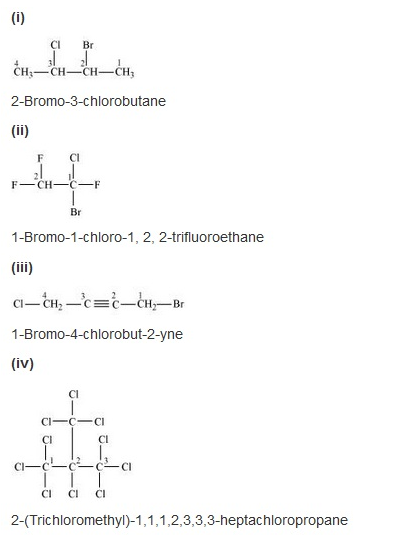
Give the IUPAC names of the following compounds:

Solution:
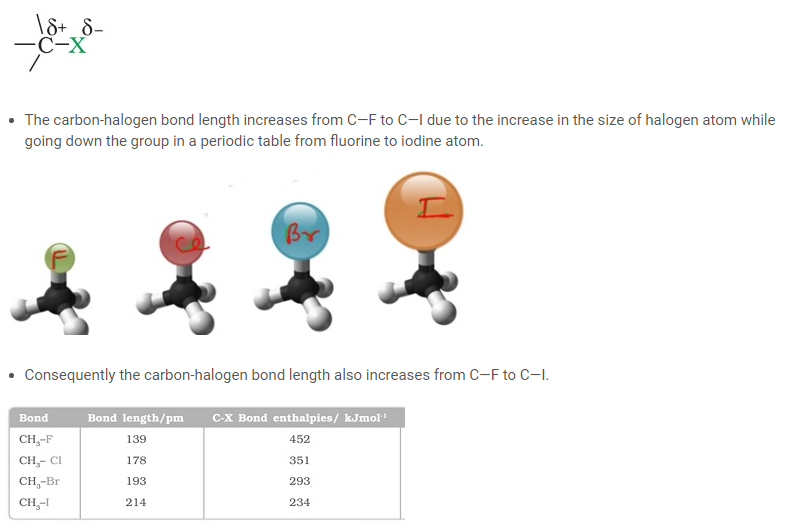
Nature of C-X Bond
- Halogen atoms being more electronegative than carbon possess a polarized bond between the carbon halogen of alkyl halide.
- The carbon atom of the alkyl halide possesses a partial positive charge. The halogen atom on the other hand possesses a partial negative charge.
Methods of Preparation of haloalkanes
- By halogenations of alkanes in presence of light, catalyst or heat:

- Allylic halogenations:
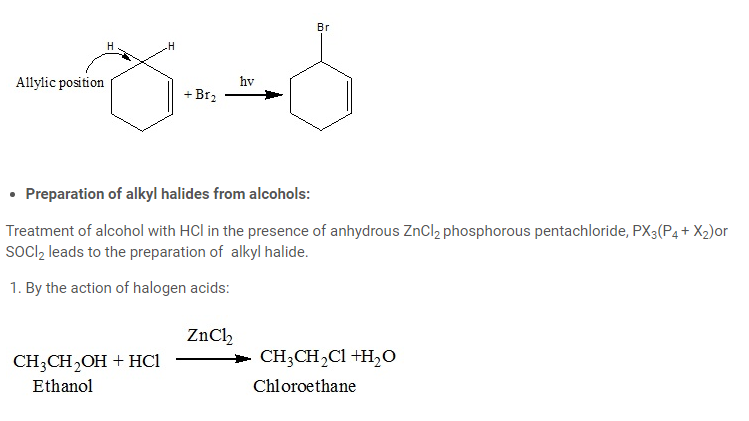
1 and 2 degree alcohols require anhydrous ZnCl2 while alcohols do not require ZnCl2. Mixture of conc. HCl and anhydrous ZnCl2 is called Lucas reagent.
Primary and secondary alkyl chlorides are prepared from their respective alcohols by using HCl gas and anhydrous ZnCl2.
- By the action of phosphorous halides:
3ROH + PX3 à 3RX + H3PO3.
Alkyl chlorides can be synthesized by the action of PCl3 or PCl5.
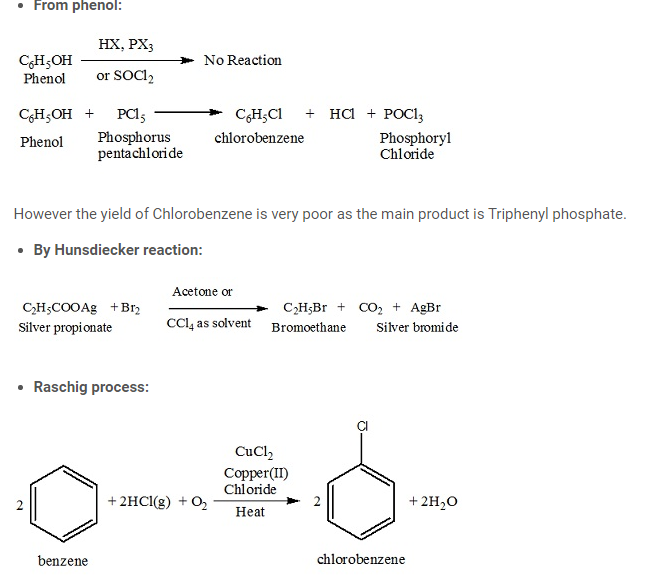
- Darzen method:
Darzens halogenation is a chemical processs involving the preparation of alkyl halides from alcohols by treating with reflux of thionyl chloride or bromide (SOX2) in the presence of small quantity of a nitrogen base like tertiary amine or pyridine, or the equivalent hydrochloride.
Alkyl bromides and iodides cannot be prepared by this method. The reason behind this is that thionyl bromide is unstable and thionyl iodide does not exist. This method is preferred for preparing alkyl chlorides because here by-products are gaseous SO2 and HCl which escape easily. But this does not happen in the method involving phosphorous chloride; hence it is not used for preparing alkyl chlorides.
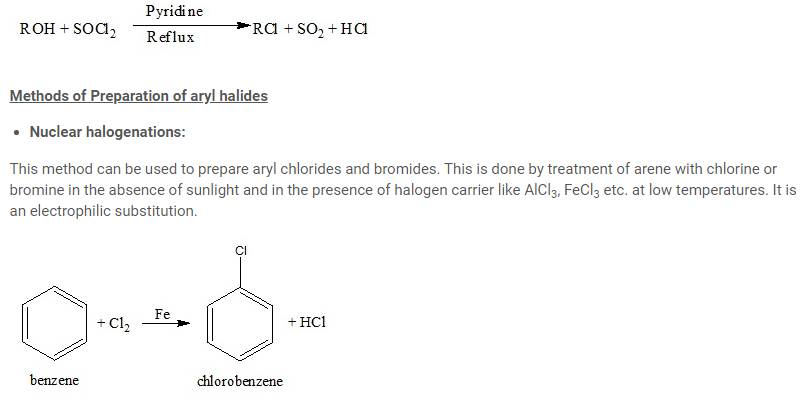
Sandmeyer reaction
Diazonium salts are highly reactive compounds used to prepare arene derivatives. Treating diazonium salt with copper (I) chloride (Cu2Cl2) or copper (I) bromide (Cu2Br2) leads to the formation of corresponding haloarene. This reaction is known as Sandmeyer reaction.

Gattermann reaction
Haloarenes can also be prepared by reaction of benzene diazonium chloride with copper powder in the presence of corresponding halogen acid. This reaction is termed as Gattermann reaction.

Physical Properties of halo alkanes
- Alkyl halides are colorless in pure state. Bromides and iodides cultivate colour on exposure to light
- Volatile halogen compounds possess a sweet smell.
- Intermolecular forces of attraction of halogen derivatives are stronger due to greater polarity and molecular mass as well compared to the parent hydrocarbon thereby resulting in higher boiling points of chlorides, bromides and iodides compared to hydrocarbons of equivalent molecular mass.
- The boiling points of alkyl halides for same alkyl group follow the order: RI> RBr> RCl> RF due to the increase in size and mass of halogen atom thereby increasing the extent of van der Waal forces.
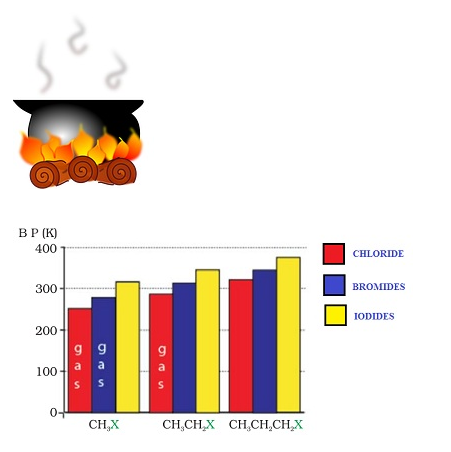
- In isomeric haloalkanes the boiling points decreases with increase in branching.

- Para-isomers possess high melting point compared to ortho and meta-isomers due to the symmetry of para-isomers fitting in crystal lattice better than ortho– and meta-isomers.
- Density increases with increase in number of carbon atoms, halogen atoms and atomic mass of the halogen atoms. Consequently the density of Bromo, iodo and polychloro derivatives of hydrocarbons is greater than water.
- The haloalkanes are only very slightly soluble in water due to release of less energy during the setup of new attractions between the haloalkane and the water molecules which in turn is insufficient to overcome the attractions between the haloalkane molecules and break the hydrogen bonds between water molecules.
- Haloalkanes dissolves easily in organic solvents due to the new intermolecular forces of attractions between haloalkanes and solvent molecules having equivalent strength as the one that is broken in separate haloalkane and solvent molecules.
Problem:
Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane.
(ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Solution:

Substitution reaction in halo alkanes
Nucleophilic substitution reactions are most common reactions of alkyl halides.
The most common nucleophiles are OH– , CN–, NO2–, SH–, NH2–, OR– and RCOO–
Following are some examples:

The tertiary alkyl halides react by SN1 mechanism via formation of carbocation as intermediate. The reactivity order for SN1 reaction is
Benzyl > Allyl > 3̊ > 2̊ > 1̊ > CH3X.
A mechanism for the reaction of tert-butyl chloride with water apparently involves two steps:

Key features:
More stable will be the carbocation intermediate; faster will be the SN1 mechanism.
Polar solvents lead to polar transition state which in turn accelerates the SN1 reaction.
If the initial compound is chiral then SN1 reaction ends up with racemization of the product.
Weaker bases being leaving group favor SN1 reaction.
SN2 mechanism
In case of SN2 reactions the halide ion leaves from the front side whereas the nucleophiles attacks from the back side; due to this reason SN2 reactions are always accompanied by the inversion of configuration. Thus formation of another enantiomer is lead by SN2 reaction of an optically active halide i.e. optical activity is retained but with opposite configuration

Key features:
In SN2 reaction the stereochemistry around carbon atom of the substrate undergoes inversion and is known as walden inversion.
The rate of reaction depends on the steric bulk of the alkyl group.
Increase in the length of alkyl group decreases the rate of reaction. Alkyl branching next to the leaving group decreases the rate drastically.
Under the following conditions SN1 and SN2 reactions take place:
- The alkyl is secondary and tertiary.
- The solvent is Protic or Aprotic.
- To stabilize the intermediate stage.
SN2 reaction and conditions required for it
Alkyl halide: SN2 reaction will be slowed down if there exists steric congestion around the carbon atom undergoing the inversion process.
CH3X > RCH2X >> R2CHX >>> R3CX
i.e. we conclude that if there is less congestion then the reaction will be faster.
Leaving group: the leaving group with a full negative charge is always expelled out. As a result the best leaving groups are those that can best stabilize an anion.
Solvent: Surroundings which the solvent surrounds can cause a reaction to go faster or slower. Solvent are of two types:
- Protic solvent: Nucleophile suffers salvation in which Protic solvent hydrogen bonds with anion that tries to attack the alkyl halide thus creating a shield around the nucleophiles. As a result reaction is slowed down. E.g. Alcohol, water
- Aprotic (polar) solvent: Aprotic solvents are those not capable of donating H’s. Due to which it’s favorable in SN2 reaction because it’s a 1step reaction and it doesn’t require forming or having H’s to stabilize the Carbocation step. E.g. Acetone, DMSO.
SN1 reaction and conditions required for it
Alkyl halide:
SN1 reaction occurs faster if the carbocation intermediate is more stable and has a lower activation barrier.
3̊ carbocation > 2̊ carbocation > 1̊ carbocation.
Leaving group:
The leaving group with a full negative charge is always expelled out. As a result the best leaving groups are those that can best stabilize an anion. In this reaction, it will not be odd to consider water as a leaving group.

Solvent:
When a carbocation undergoes solvation it allows the carbocation to get surrounded by more electron density thereby making the +ve charge more stable. The solvent can be protic or Aprotic but it must be polar.
Nucleophiles:
Those nucleophiles that undergo SN1 mechanism are weak neutral molecules. Any nucleophiles stronger than this is also a strong base that results in elimination reactions.
Elimination reactions:
This reaction involves the loss of two atoms or groups from the substrate as by product with formation of pi-bond. A halogen along with a hydrogen atom is removed from adjacent carbon atoms to form a double bond. This elimination introduces multiple bonds. It can be classified into E1 and E2 reaction.
E1 reaction
It is a unimolecular reaction. Rate determining step consist of formation of carbocation intermediate. Stability of carbocation intermediate determines the reactivity of E1 reaction.
Order of reactivity for E1 reaction is 30 > 20 > 10. Both elimination and substitution reaction involves the use of (same reactive intermediate) carbocation. Therefore both the products are formed in comparable amount. This reaction is favored by entropy of reaction therefore increase in temperature favors the E1 reaction.
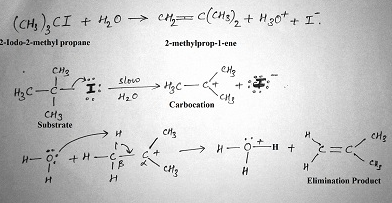
Stereochemistry of E1 reaction:
E1 eliminations generally lead to the more stable stereochemistry.
The rate of the E1 reaction depends only on the substrate, therefore more stable the carbocation is, faster will be the reaction. Slowest step is the formation of the carbocation. Alkenes formation doesn’t require strong base, since there is no leaving group that needs to be displaced. So there is no requirement for the stereochemistry of the starting material;
E2 reaction
It’s a biomolecular reaction. It is a single step reaction whose rate depends on the concentration of base and substrate. Reactivity depends on both strength of base and nature of alkyl halide. Order of reactivity for E1 reaction is 30 > 20 > 10. This reaction proceeds at room temperature.
Example:
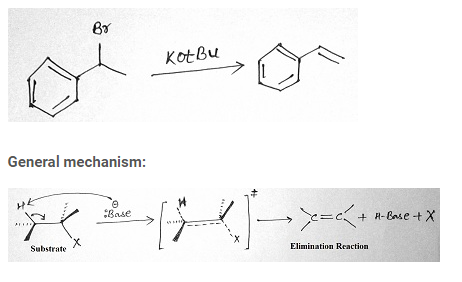
Stereochemistry of E2 reaction:
E2 eliminations may or may not lead to the more stable stereochemistry. Initial material for this reaction has two sp3 hybridized carbons which on rehybridization forms two sp2 hybridized carbons. The C-X bond and the C-H bond lines up in the same plane and faces in anti directions to each other.

Conditions of E2 reaction:
The conditions favoring SN2 reaction favors E2 reactions which are as follows:
Alkyl halide: SN2 reaction will be slowed down if there exists steric congestion around the carbon atom undergoing the inversion process.
i.e. we conclude that if there is less congestion then the reaction will be faster.
CH3X > RCH2X >> R2CHX >>> R3CX
Leaving group: the leaving group with a full negative charge is always expelled out. As a result the best leaving groups are those that can best stabilize an anion.
Solvent: Surroundings which the solvent surrounds can cause a reaction to go faster or slower. Solvent are of two types:
- Protic solvent: Nucleophile suffers salvation in which protic solvent hydrogen bonds with anion that tries to attack the alkyl halide thus creating a shield around the nucleophiles. As a result reaction is slowed down. E.g. Alcohol, water
- Aprotic (polar) solvent: Aprotic solvents are those not capable of donating H’s. Due to which it’s favorable in SN2 reaction because it’s a 1step reaction and it doesn’t require forming or having H’s to stabilize the Carbocation step. E.g. Acetone, DMSO.
Saytzeff’s rule of elimination:
According to this rule although alkene synthesis leads to the formation of more than one product, the more substituted alkene is the major product.
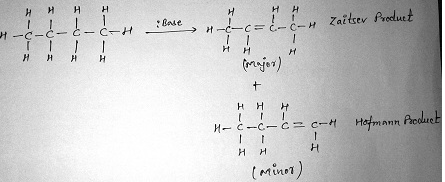
Conditions of E1 reaction:
The factors that favors SN1 reaction also favors E1 reaction which are as follows:
Alkyl halide:
E1 reaction occurs faster if the carbocation intermediate is more stable and has a lower activation barrier.
3̊ carbocation > 2̊ carbocation > 1̊ carbocation.
Leaving group:
The leaving group with a full negative charge is always expelled out. As a result the best leaving groups are those that can best stabilize an anion. In this reaction, it will not be odd to consider water as a leaving group.
Solvent:
When a carbocation undergoes solvation it allows the carbocation to get surrounded by more electron density thereby making the +ve charge more stable. The solvent can be protic or Aprotic but it must be polar.
Nucleophiles:
Those nucleophiles that undergo E1 mechanism are weak neutral molecules. Any nucleophiles stronger than this is also a strong base that results in elimination reactions.
Like the E2 reaction, the E1 favors the Zaitsev product (always).
Comparison of SN1, SN2, E1 and E2
- E1 and E2
E2 reaction is favored by strong base whereas strength of base doesn’t determine whether the reaction will be favored or not.
Order of reactivity is same for both the reaction i.e. 3̊ > 2̊ > 1̊ .
E1 reaction is favored by polar protic solvent because it accelerates the reaction by solvating halide ion forming carbonium ion whereas E2 reaction is decelerated by polar protic solvent.
E2 reaction is favored at room temperature whereas E1 reaction requires little higher temperature.
- SN2 and E2
High concentration of a strong nucleophiles or base favors both the reactions.
Increase in temperature favors elimination over substitution. They are entropically favored over substitution due to the fact that products of elimination are greater in number than the reactants and increase in temperature increases the entropy effect. This is due to the relation ΔG0 = ΔH0 – TΔS0.
Another way to influence the elimination reaction is to strong sterically hindered base. In the reaction mentioned below E2 reaction is 85 % whereas SN2 is 15%

- SN1 and E1
E1 reactions are favored by the use of substrates that can form stable carbocation. They are also favored by the use of polar solvents. At lower temperature SN1 is favored over E1 reaction.
Strong nucleophile & Weak Nucleophile
Strong nucleophile
Usually anions with a full negative charge and no steric hindrance. Nucleophilicity increase with increase in charge density. For example, NaOR, RLi, NaOH or KOH, NaCN or KCN, NaCCR, NaNH2, NaNHR, NaI, LiBr, KI, NaN3.
Weak Nucleophile: Neutral. For example,
Basicity and Nucleophilicity essentially describe same phenomenon, except basicity concerns donation of lone pair of hydrogen and Nucleophilicity concerns donations of lone pair to all other atoms.
Organometallic Compounds
Most organic chlorides and iodides react with certain metals to give compounds containing carbon-metal bonds called organo-metallic compounds.
For example, RMgX is referred to as Grignard Reagent in 1900 by Victor Grignard.
Carbon-magnesium n=bond is covalent but highly polar.
Grignard reagents are highly reactive and react with any source of proton to give hydrocarbons.
Chemical properties:
It involves
- Nucleophile Substitution
- Elimination Reaction
- Reaction with metals
Nucleophilic Substitution: Resonance effect
Aryl halides are extremely less reactive towards Nucleophilic substitution reactions. Let us now discuss resonance effect.

Nucleophilic Substitution:sp2 hybridised
C-X bond in halo alkane is 177 Pico meter. Whereas C-X bond in haloarenes is 169 Pico meter. Therefore the C-x bond in haloarenes is strong and stable and hence difficult to break. Hence the Nucleophilic substitution in haloarenes is difficult.
Nucleophilic Substitution: unstable cation
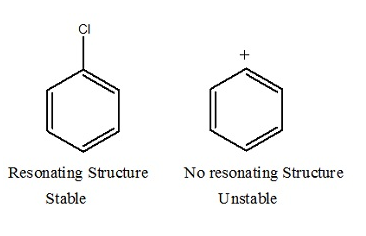
In figure II resonance structure is not possible. Cation formation is not possible as the cation is unstable.
This shows that SN1 is totally ruled out.
SN2 is possible.
Polyhalogen Compounds
Dichloromethane (Methylene chloride)

- It is used as solvent, paint remover, propellant in aerosols, process solvent in the manufacture of drugs.
- It is used as metal cleaning and finishing solvent.
- But human beings can be adversely affected when exposed to Methylene
- It causes harm to human mental health.
- Human exposure to even lower levels of mythlene chloride in air can lead to dizziness, nausea, tingling, numbness in fingers and toes, etc.
- Direct exposure to Methylene chloride can cause intense burning and mild redness in the skin.
- Cornea of eyes can be adversely burnt on direct exposure to Methylene
Trichloromethane (Chloroform)

- Chloroform is a sweet smelling, heavy and colorless liquid. It has low B.P. of 61o
- It is insoluble in water but soluble in organic solvents.
- If it is inhaled it causes unconsciousness.
- It is used as anesthetic because when pure chloroform is inhaled it affects the heart due to which after mixing with ether and other suitable anesthetics chloroform can be used as anesthetic.
- Chloroform on oxidation in air leads to the formation of phosgene which is a poisonous gas due to which it should be stored in a dark colored bottle.
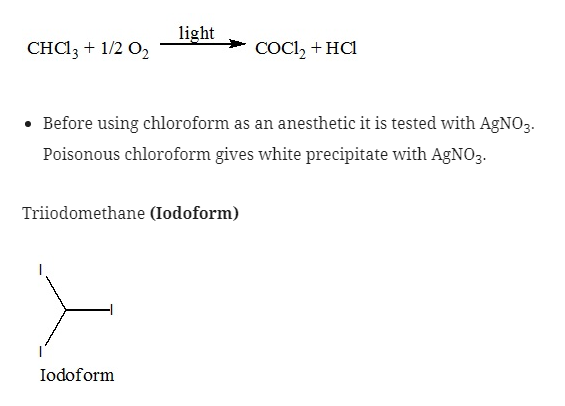
- They are used as an antiseptic due to the liberation of free iodine. It is not because of Iodoform itself.
- But due to the offensive smell it was replaced by some other solutions that contain iodine.
Tetra chloromethane (Carbon tetrachloride)
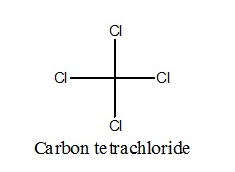
- They are used in manufacturing refrigerants and propellants for aerosol cans.
- They are also used for the synthesis of chlorofluorocarbons, pharmaceutical etc.
- It was extensively used as cleaning agent in industry and as a degreasing agent at home as well.
- It is also used as a spot remover and fire extinguisher.
- Exposure to CCl4 can adversely affect the heart beat and make it beat irregularly or make it permanently stop.
- Exposure to eyes can cause irritation.
- Exposure to atmosphere can lead ozone depletion that may lead to rise in the level of exposure to ultraviolet rays. This in turn leads to increased risk of skin cancer, eye diseases and other disorders as well as weakened immune system.
Freons
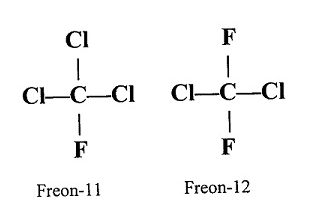
- The chlorofluorocarbon compounds of methane and ethane are jointly called freons.
- They are very stable, non-corrosive, non-toxic, and unreactive liquefiable gases.
- Freon 12 (CCl2F2) is most commonly used Freons in industrial sector.
- Freons are manufactured from tetra chloromethane using Swarts reaction.
- Freons are extensively used in aerosol propellants, refrigerants and air conditioners.
p,p’-Dichlorodiphenyltrichloroethane(DDT)

- DDT stands to be the first chlorinated organic insecticides originally discovered in 1873 which was then further studied and it was 1939 when Paul Muller discovered the effectiveness of DDT as an insecticide.
- It is highly poisonous to all living organisms as it does not get metabolized rapidly by animals and gets deposited and stored in the fatty tissues.
Problem:
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Solution:

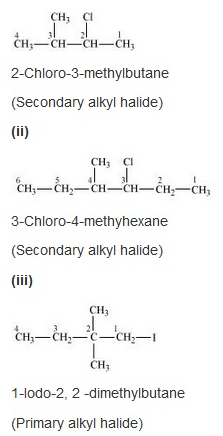
Problem:
Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
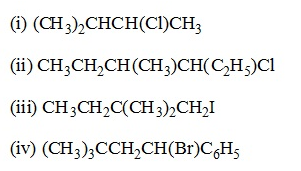
Solution:

Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]






