Chemistry Chapter 10 Haloalkanes and Haloarenes NCERT Solution
Haloalkanes And Haloarenes Class 12 NCERT Solutions – INTEXT Questions
Question 1. ( Haloalkanes and Haloarenes )
Write the structures of the following compounds :
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcydohexane
(iii) 4-terf-Butyl-3-iodoheptane
(iv) 1, 4-Dibromobut-2-ene
(v) 1 -Bromo-4-sec-butyl-2-methylbenzene
Solution:

Haloalkanes And Haloarenes Class 12 NCERT Solutions
Question 2.
Why is sulphuric acid not used during the reaction of alcohols with Kl ?
Solution:
H2SO4 is a strong oxidising agent. Therefore, when it is used in presence of KI, it tends to convert KI to HI and finally oxidises it to I2.

Question 3.
Write structures of different dihalogen derivatives of propane.
Solution:
The structures of all possible dihalogen derivatives of propane are
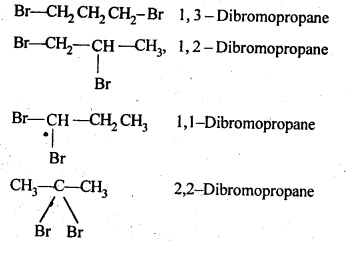
Haloalkanes And Haloarenes Class 12 NCERT Solutions Question 4.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields
(i) A single monochloride
(ii) Three isomeric monochlorides
(iii) Four isomeric monochlorides.
Solution:
An alkane with molecular formula C5H12 can exist in the following isomeric forms :
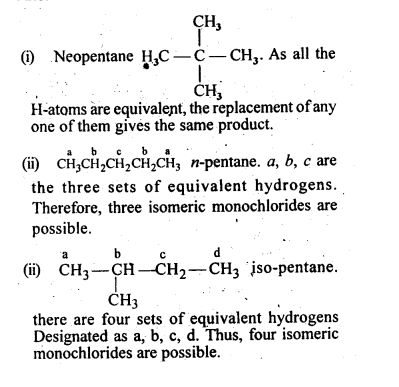
Question 6. ( Haloalkanes and Haloarenes )
Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, bromoform, chloromethane, dibromomethane.
(ii) 1 – Chloropropane, isopropyl chloride, 1 – chlorobutane.
Solution:
The boiling points of organic compounds depend on the strength of the intermolecular forces in them. These forces are :
(a) van der Waals forces and
(b) dipole-dipole interactions These forces are dependent on the
(i) molecular mass and
(ii) surface area of the molecules
(i) As the molecular mass of the compound increases, the boiling point also increases. Therefore the correct order is
chloromethane < bromomethane < dibromomethane < bromoform
(ii) Amongst molecules with same mass, it is the size of the molecule that determines the boiling point. Branched compounds are more compact and therefore have less surface area as compared to their straight chain counterparts and therefore lower boiling point. The order of boiling point is
iso-propyl chloride < 1-chloropropane < 1-chlorobutane
Question 7. ( Haloalkanes and Haloarenes )
Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
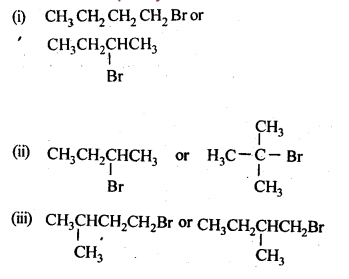
Haloalkanes And Haloarenes Class 12 NCERT Solutions:
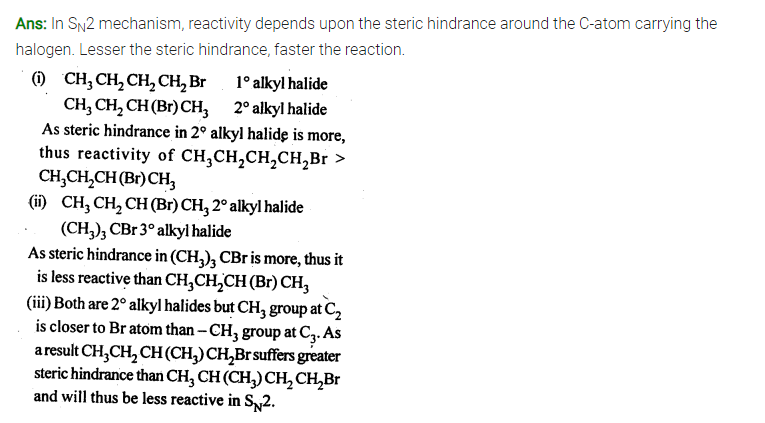
Question 8. ( Haloalkanes and Haloarenes )
In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?
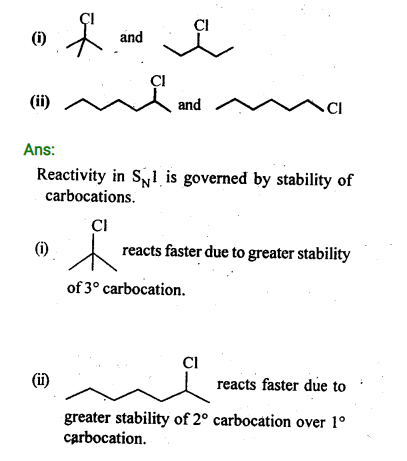
Ans

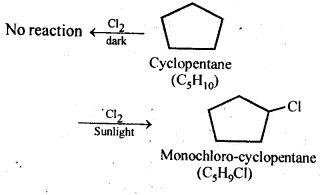
10.10. A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Ans: The hydrocarbon with molecular formula C5H10 can either a cycloalkane or an alkene. Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]






