www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Engineering post, Career Guidelines , English Speaking Trick , How to crack interview and lots more. ( P Block Elements )
P Block Elements Notes for Exam
Group 15
The group 15 members are:
- Nitrogen (N)
- Phosphorous (P)
- Arsenic(As)
- Antimony (Sb)
- Bismuth (Bi)
Group 15
The group 15 members are:
- Nitrogen (N)
- Phosphorous (P)
- Arsenic(As)
- Antimony (Sb)
- Bismuth (Bi)
Physical Properties of group 15:
- Electronic configuration of group 15 members: general electronic configuration is ns2np3.
- Nitrogen (N) [He]2s2,2p3
- Phosphorous (P) [Ne]3s2,3p3
- Arsenic(As) [Ar]3d10,4s2,4p3
- Antimony (Sb) [Kr]4d10,5s2,5p3
- Bismuth (Bi) [Xe]4f14,5d10,6s2,6p3
- Atomic size: As compared to group 14 they are smaller in size due to increased nuclear charge.
Along group, size increases as every time a new shell is being added due to which nuclear charge decreases.
- Ionization energy: The group 15 has high ionization energy than group 14 because of smaller size.
Along group, ionization energy decreases as size increases
- Electro negativity: Because of smaller size the group 15 members re more electronegative than group 14. Out of them the increasing order of electro negativity is:
N>P>As>Sb>Bi
- Metallic character: They are less metallic than group 14 because of small size and increased nuclear charge.
Along group metallic character increases as size increases and ionization energy decreases. The order of their metallic character is: N<P<As<Sb<Bi
- Melting point and boiling point: The melting point depends upon the type and number of bonds formed whereas boiling point depends upon Vander wall force which increases in magnitude with increase in size.
- Boiling points: It increases down the group as size increases. The order is – N<P<As<Sb<Bi
- Melting point: It first increases then decreases.
The order is: N<P<As>Sb>Bi
The reason for this decrease in case of antimony and bismuth is due to use of only three electrons out of 5 in bond formation because of inert pair effect.
- Catenation : They show only up to small extent like P exist as P4
Nitrogen as N2.
- Oxidation states: They have configuration ns2np3. Their common oxidation states are +5 and +3.
The oxidation states shown by them are:
- Nitrogen shows: –
- -3 (Ca3N2) Calcium Nitride
- -2 (NH2NH2) pyridine
Also show +3.
- Phosphorous shows:
-3 (Ca3P2) Calcium Phoshide
- Bismuth: as +3 due to inert pair effect. It has only one compound in +5 oxidation state that is BiF5 (Bismuth pentafluoride).
Please note: Nitrogen do not form compounds in +5 oxidation states because:
- It has no vacant d orbital therefore no excitation can occur. So, maximum covalence shown by it is 4.
Anomalous behavior of nitrogen:
- It has small size
- It has high ionization energy
- It is most electronegative
- It has no vacant d orbital
- In it no dπ-pπ bonding can occur.
Chemical properties of group 15:
- Reaction with hydrogen: Whenever any substance reacts with hydrogen they form respective hydrides.
Like whenever 15 group elements react they form hydrides of order EH3 that is:
NH3 PH3 AsH3 SbH3 BiH3
Ammonia Phosphine Arsine Stibine Bismuthine
In this NH3 is called ammonia and PH3 is called phosphine.
All these hydrides are called Lewis bases.
These can be formed by reacting metal hydrides with water that is:
Mg 3N2 + H2O –>Mg(OH)2 + NH3
(Magnesium nitride) (magnesium hydroxide)( ammonia)
Likewise for preparing phosphine we take calcium phosphide:
Ca3P2 + H2O –> Ca (OH)2 + PH3
(Calcium phosphide) (calcium hydroxide) (phosphine)
Ammonia can be industrially prepared by Haber’s process:
In this nitrogen and hydrogen are taken in 1:3 ratios and catalyst Fe and MoO is used. This reaction can take place at conditions of low temperature and high pressure. The structure of these hydrides is pyramidal and hybridization sp3.
The properties of hydrides are:
- Bond angle: The bond angle decreases down the group. The order of decrease of angle is:
NH3 > PH3 > AsH3 > SbH3 > BiH3
The reason behind this is that when size of central atom increases, lone pair will push closer to bond pair-bond pair. Due to this bond angle decreases.
- Basic character: It refers to ability of molecule to donate its lone pair .the order of increase in basic character is :
NH3 > PH3 > AsH3 > SbH3 > BiH3
Most basic least basic
Due to large size of bismuth the lone pair density is less closer to bismuth therefore tendency to lose electron decreases.
- Stability: The stability of hydrides depends upon the comparability of size. The order of stability of hydrides is:
NH3 > PH3 > AsH3 > SbH3 > BiH3
Due to increase in size, bond length increase due to which bond dissociation energy decrease therefore stability decrease.
- Reducing nature: The order of reducing character of hydrides is:
NH3 < PH3 < AsH3 < SbH3 < BiH3
Out of them in case of BiH3, the bond strength is low therefore reducing character is lowest.
- Boiling point: The boiling point depends upon Vander wall force and this Vander wall force increases with increase in size.
The order of their boiling points for 15 group hydrides is:
NH3 > PH3 < AsH3 < SbH3 < BiH3
In case of NH3 and PH3, ammonia has higher boiling point than phosphine because of hydrogen bonding.
- Solubility: The solubility of hydrides in water for group 15 is:
NH3 > PH3 > AsH3 > SbH3 > BiH3
Ammonia has higher solubility due to formation of hydrogen bonds.
- Reaction with oxygen: Whenever group 15 elements react with oxygen they form oxides of order E2O3 and E2O5.
E2O3– trioxides
E2O5. – Penta oxides
Nitrogen form maximum types of different oxides ranging from +1 to +5.
The oxides of nitrogen are:
- N2O (laughing gas ) oxidation state +1
- NO oxidation state +2
- N2O3 oxidation state +3
- N2O4 oxidation state +4
- N2O5 oxidation state +5(most acidic)
In all the oxides pπ-pπ bonding takes place.
- P-forms oxide in oxidation state of +3 (P4O6), +4 (P4O8), +5 (P4O5)
- As forms oxide as As2O3, As2O5 (Arsenic trioxide and Arsenic pentaoxide).
- Sb forms oxide as Sb2O3 and Sb2O5 (Antimony trioxide and Antimony pentaoxide).
- Bi forms oxide as Bi2O5 and Bi2O3 (Bismuth trioxide and Bismuth pentaoxide).
Nature of their oxides:
Nitrogen and phosphorous forms acidic oxides.
Arsenic and antimony forms amphoteric oxide.
Bismuth forms basic oxide.
“Higher is the oxidation state more acidic is the oxide”
- Reaction with halogens
The group 15 elements combine with halogen to form their respective halides of general formula EX3 and EX5.
Nitrogen does not form pent halides due to non availability of d orbital but form trihalides.
The structure of trihalides is distorted tetrahedral of hybridization sp3.
The geometry is given below:
Nitrogen trichloride
Characteristics of their trihalides:
- They are covalent and down the group ionization increase
- Trihalides of nitrogen are least stable
- Nitrogen triflourides are maximum stable
- The order of stability of nitrogen halides are :
The reason behind is incomparable sizes.
- Trihalides of phosphorous arsenic and antimony are good Lewis bases.
- Down the group trihalides of group 15 stability increases due to inert pair effect.
Characteristics of pentahalides:
- Their general formula is EX
- Their hybridization is sp3
- The geometry of pentahalides is trigonal bipyramidal as shown below:
Phosphorous pentachloride
- Down the group stability of pent halides decreases due to inert pair effect.
- All pent halides are Lewis acids.
Please note that: The existence of PCl5 (phosphorous pentachloride)is gas but in solid state it exist as dimer {[PCl4]+[PCl6]–}
Dinitrogen
Introduction
The molecular formula is N2.
It was discovered by Daniel Rutherford in 1772.
- It is a diatomic gas.
- The triple bond that exists between nitrogen of molecule is quite strong therefore bond dissociation energy is high.
- It is an inert gas.
- Nitrogen is smallest in its group.
- Nitrogen is most electronegative.
- Nitrogen has highest ionization energy in its group.
- Nitrogen has no vacant d orbital.
Preparation of DiNitrogen
- It is prepared by reacting ammonium chloride with sodium hydroxide that is:
NH4Cl + NaOH –> NaCl + N2 + H2O
Ammonium chloride soda lime sodium chloride
This nitrogen so formed has impurities nitric oxide and nitric acid. So, in order to remove them we have to bubble this nitrogen through concentrated sulphuric acid.
- Pure nitrogen can be prepared by heating Barium nitrate :
Ba(NO3)2 —> Ba + N2 + O2
Barium nitrate Barium Nitrogen
(On heating)
Physical properties of nitrogen
- It is colorless, odorless and tasteless gas.
- It is slightly soluble in water.
- It exists as N
- It is non toxic gas.
Chemical properties of nitrogen
- Reaction with metal: Nitrogen reacts with metal to form metal nitrides.
Ca + N2 –> Ca3N2 ( Calcium Nitride)
- Reaction with non metal: They react with non metal to form compounds like:
N2 + O2 –> NO2 (Nitrogen dioxide)
N2 + H2 –> NH3 ( Ammonia)
Uses of nitrogen:
- Liquid nitrogen is used as preservative for specimens.
- It is used to manufacture compounds like nitric acid etc.
- It is used to create inert atmosphere.
Ammonia
The molecular formula of ammonia is NH3.
Preparation:
Lab preparations:
- Hydrolysis of urea: The reaction involved is given below that is
- By reacting ammonium chloride with calcium hydroxide:
- By reacting ammonium sulphate with sodium hydroxide:
Industrial preparation:
In this nitrogen and hydrogen are taken in 1:3 ratios and catalyst Fe and MoO is used. This reaction can take place at conditions of low temperature and high pressure. The structure of these hydrides is pyramidal and hybridization sp3.
N2 + H2 (It is a irreversible reaction) <—> 2NH3
nitrogen hydrogen ammonia
The reaction takes place at low temperature, high pressure, catalyst Fe, catalytic promoter MoO (molybdenum oxide).
Physical properties of ammonia
- It is colorless gas with pungent smell.
- It is highly soluble in water forming ammonium hydroxide.
- It can be easily liquefied.
- It has high boiling point due to hydrogen bonding.
Chemical properties of ammonia
- Basic nature: It behaves as a Lewis base because of lone pair of electrons. Also it has tendency to behave as bronsted base that is :
Both the reactions above is neutralization reactions
- It behaves as weak base that means it precipitates hydroxides.
- The lone pair present helps in forming complexes so we can say it is a complexion agent.
This complexing nature of ammonia is due to the lone pair present in it so, it can act as electron donor.
Uses of ammonia
- Ammonia helps in making nitrogenous fertilizers like ammonium sulphate etc.
- It is used in manufacture of nitric acid.
- The liquid ammonia is used as refrigerant.
Oxides of nitrogen
The nitrogen forms variety of oxides that are given below:
- N2O (laughing gas ) oxidation state +1
- NO oxidation state +2
- N2O3 oxidation state +3
- N2O4 oxidation state +4
- N2O5 oxidation state +5(most acidic)
- N2O: nitrous oxide or laughing gas
- In it the oxidation sate of nitrogen is +1.
Preparation:
- It is prepared by heating ammonium nitrate that is:
- NH4NO3 (on heating) –> N2O + H2O
- (Ammonium Nitrate) Nitrous Oxide Water
- The laughing gas so formed is colourless, neutral and exist in gaseous state.
- It structure is :
N– = N+ = O
Nitrous oxide
2. NO (nitric oxide )
- In it the oxidation state of nitrogen is +2.
Preparation:
- The NO formed is colorless and neutral gas.
- The structure of nitric oxide is given below:
Nitric oxide
- N2O3 (nitrogen trioxide)
- In it the oxidation state is +3.
Preparation:
- NO + N2O4 –>N2O3
- This nitrogen trioxide is blue solid and acidic in nature.
- The structure of it is:
Nitrogen trioxide
- NO2 (Nitrogen dioxide )
- In it the oxidation state of N is +4.
Preparation:
Pb(NO3)2 (ON heating)—> PbO + NO2 + O2
Lead Nitrate LeadOxide Nitrogen dioxide
- The nitrogen dioxide so formed is brown gas with acidic nature.
- The structure of it is:
Nitrogen dioxide
This single electron system in it helps it in existing as dimer (N2O4).
2NO2 <—> N2O4
Nitrogen dioxide Dimer of Nitrogen dioxide
On cooling we get dimer and on heating dimer we get nitrogen dioxide.
- N2O5 (nitrogen pent oxide )
- In it the oxidation state is +5.
- This is most acidic among all oxides of nitrogen.
Preparation:
This nitrogen pent oxide is colorless gas with acidic nature.
- The structure of this nitrogen pent oxide is given below:
Nitrogen pentaoxide
Nitric acid (HNO3)
Preparation:
- Industrially : It is prepared from Ostwald’s process
The reaction involves various steps:
The nitric acid so prepared is 98% pure rest 2% can be purified by concentrated sulphuric acid.
Physical properties of nitric acid
- It is colorless liquid.
- The impure nitric acid is yellow because of nitrogen dioxide in it as impurity therefore nitric acid is called as fuming nitric acid also.
- It is corrosive in nature.
Chemical properties of nitric acid
It keeps on oxidizing and forms nitrogen dioxide that is why we see brown color fumes coming out from the bottle.
- Acidic nature: Nitric acid is acidic in nature because it dissociates to give hydrogen ion when dissolved in water:
Because this is acidic therefore, it reacts with base showing neutralization reaction:
HNO3 + NaOH —> NaNO3 + H2O
Nitric Acid Sodium Hydroxide Sodium Nitrate Water
- Reaction with metals: To give nitrates and nascent hydrogen:
M + HNO3 —> metal nitrates + [H]+
This nascent hydrogen can bring about reduction of substance:
Example:
Please note certain metals like chromium, aluminium do not dissolve in nitric acid due to formation of passive layer of oxide film on it which prevents further reaction.
Aqua regia: It is a mixture of nitric acid and hydrochloric acid in ratio 1:3 and it is used in dissolving noble metals.
- Oxidizing nature of nitric acid: It helps in oxidation of non metals like for example:
- Converting I2 –> IO3–
I2 — >IO3–
In this oxidation state of iodine is changed from 0 to +5 that is increase in oxidation number:
Test for nitrates
It is also called as brown ring test.
In this we take test tube and sample (salt containing nitrate) to it.
- Then slowly pour concentrated sulphuric acid to it.
- Then we put solution of ferrous sulphate and allow it to stand.
- Then on interface a brown ring is formed that shows the presence of nitrates.
- The ring that is formed is chemically [Fe(H2O)5(NO)]2+(Nitroso ferrous sulphate ).
Uses of nitric acid:
- In manufacturing of ammonium nitrate and other fertilizers.
- In manufacturing of explosives like TNT (trinitrotoluene).
- In purification of gold and silver by using aqua regia.
- It is used as oxidizer in rocket fuels.
Formation of oxy-acids
(a)Oxy-acids of nitrogen: nitrogen forms four types of oxy-acids that is:
- Nitric acid (HNO3)
- Nitrous acid (HNO2)
- Hypo nitrous acid (H2N2O2)
- Per nitric acid (HNO4).
Nitrous acid: Nitrous acid oxidizes H2S to S ,KI to I2 and act as an oxidizing agent. In the presence of strong oxidizing agent it also acts as reducing agent like it reduces potassium dichromate etc to form complex.
It structure is:
Nitrous acid
Nitric acid:
- It stains skin yellow due to formation of nitro compound xanthoprotein. It is also called as aqua Fortis meaning strong water because it attacks almost all metals.
- It also forms a constant boiling mixture with water which has 68% of acid.
- Concentrated nitric acid attains a yellow color on standing. This is due to the presence of nitrogen dioxide which is formed due to decomposition of nitric acid in sunlight. This yellow color may be removed by warming it or bubbling air through it.
- Fuming nitric acid contains nitrogen dioxide in concentrated nitric acid. It is obtained by distilling concentrated nitric acid with little amount of starch.
- It structure is:
Nitric acid
Hypo nitrous acid:
- It is a weak dibasic acid
- Its structure is:
Hypo nitrous acid
Oxy-acids of phosphorous: Its oxy acids are:
- Hypo phosphorous acid (H3PO2)
- Phosphorous acid (H3PO3)
- Orthophosphoric acid (H3PO4)
- Metaphosphoric acid (HPO3)
- Hypo phosphoric acid (H4P 2 O6)
- Pyro phosphoric acid (H4P2 O7)
- Pyro phosphorus acid (H4P2 O5)
- Isohyphosphoric acid (H4P2 O6)
Their structures are given below:
Hypo phosphoric acid
- It is also called as phosphinic acid.
- It is powerful reducing agent because of the presence of two P-H bonds.
- The oxidation state of P is +1(monobasic).
Phosphorous acid
- It is also called as phosphonic acid.
- It is dibasic acid.
- In it the oxidation state of P is +3.
- It is strong reducing agent but weaker than hypo phosphorous acid due to only one P-H bond.
- It undergoes disproportionate reaction giving orthophosphoric acid and phosphine.
Orthophosphoric acid
- It is a weak tri basic acid.
- In it the P is in +5 oxidation state.
- It has no oxidizing or reducing properties.
- On heating it gives pyro phosphoric acid at 525K and meta phosphoric at 875K.
H3PO4àH4P2O7 àHPO3
(At 525K) (At 875K)
Metaphosphoric acid
- It is also called as glacial phosphoric acid.
- It is monobasic having P in +5 oxidation state.
- It has no reducing property.
- It exists as polymer and thus contains P-O-P bonds which can further form calgon.
Hypo phosphoric acid
- It is tetra basic in nature.
- Due to absence of P-H bond it has no reducing properties.
- In the P is in +4 oxidation state.
Pyro phosphoric acid
- It is also called as di phosphoric acid.
- In it the oxidation state is +5.
- It is also tetra basic.
- It has no reducing property.
Pyrophosphorous acid
- It is also called as di phosphorous acid.
- In it the oxidation state of P is +3.
- It contains P-O-P bond and is dibasic.
- It is strong reducing agent due to presence of P-H bonds.
Isohyphosphoric acid
- It contains one P-O-P bond and one P-H bond.
- It is also called as diphosphoric acid.
- It is tri basic in nature in it the oxidation state of P is +3 which are attached to hydrogen and rests have +5 oxidation state.
- It has reducing properties also.
Phosphorous
It exists as P4 with tetrahedral geometry as shown below:
Phosphorous
It has three forms:
- White P
- Black P
- Red P
White phosphorous
- It has a normal tetrahedral structure. They are soft waxy solids.
- They can be cut with knife.
- They are poisonous.
- They are chemiluminescent.
- It is most reactive form of phosphorous.
- It undergoes disproportionate reaction as shown :
- P4 + NaOH —> PH3 + NaH2PO2
- Phosphorous Sodiumhydroxide Phosphine Sodiumhypo Phosphite
- In this oxidation sate of phosphorous changer from 0 to +1 and -3.
Black phosphorous
It exists in two forms:
Alpha phosphorous
Beta phosphorous
Alpha phosphorous: is formed by heating red phosphorous at 803 K
Beta phosphorous: is formed by heating white phosphorous at 473K
It has most stable due to three dimensional structure as shown:
Alpha phosphorous
Red phosphorous
- It is obtained by heating white phosphorous at 573 K in inert atmosphere.
- It has polymeric structure as shown:
Red phosphorous
- It is crystalline solid.
- It shines grey.
- It is non poisonous.
- It is more stable.
- It is also chemiluminescent.
- Stability of different forms of phosphorous:
White P< red P <black P
Phosphine
Its molecular formula is PH3
Its structure is:
Phosphine
Preparation
It is prepared by dropping water on calcium phosphide.
Lab preparation: By disproportionate reaction
This phosphine produced can be purified by reacting it with HI to get PH4I and then this PH4I react with KOH to form pure PH3 ,KI and water.
Physical properties:
- It is colorless gas with smell of rotten fish.
- It is poisonous.
- It is slightly soluble in water.
- The solution of phosphine is unstable and decomposes in presence of light.
Chemical properties
- It is weakly basic due to lone pair of electrons.
- It undergoes precipitation reaction that is:
Uses of phosphine:
(a) Combustion of PH3is used in Holmes’s signals for ships.
(b)It is also used as smoke screens in warfare.
Introduction of group 16
The members of this family are:
- Oxygen (O)
- Sulphur (S)
- Selenium (Se)
- Tellurium(Te)
- Polonium (Po)
Physical properties of group 16
- The electronic configuration for them are : ns2 np4
- Oxygen (O) [He]2s2,2p4
- Sulphur (S) [Ne]3s2,3p4
- Selenium (Se) [Ar]3d104s24p4
- Tellurium(Te) [Kr]4d10,5s2,5p4
- Polonium (Po) [Xe]4f14,5d10,6s2,6p4
- Atomic size: It increases down the group as every time a new shell is added as we move down. If we compare size of group 16 elements with group 15 then we see that group 16 is smaller due to increased nuclear charge.
- Ionization energy: If we compare ionization energy of group 16 and group 15 we see, that group 16 has lower energy than group 15 because of half filled stable electronic configuration of group 15 i.e. ns2np3.
As we move down the group, the ionization energy decreases because the size increases down the group.
- Electro negativity: The group 16 elements are more electro negative than group 15 because of small size of group 16 elements. This group has second highest electro negativity in whole periodic table.
- Oxygen is second most electronegative in periodic table.
- Down the group electro negativity decreases as size increases.
- Oxidation states: They generally show -2 oxidation state.
- For oxygen: Common oxidation state is -2 but oxygen also show +2 oxidation state like in case of OF2 and in O2F2 the oxidation state is +1. The oxidation state of oxygen is in +ve because oxygen is less electronegative than fluorine.
- For sulphur: The oxidation state shown is +2, +4, +6 (because of vacant d orbital). Like in SO2 the oxidation state of sulphur is +4 and in sulphuric acid the oxidation state is +6 and in H2S it is -2.
- Down the group the vacant d orbital are present therefore they all can show +2, +4, +6 oxidation states.
Anomalous behavior of oxygen:
- It has smallest size in its family.
- It has highest ionization energy.
- No vacant d orbital is present in it.
- It is highly electronegative in its family.
- Electron gain enthalpy
Along period it is more negative because of attraction towards electron because of increased nuclear charge.
Down the group electron gain enthalpy keeps on becoming less negative because nuclear charge decreases and size increases.
The trend that was expected
- O>S>Se>Te (wrong trend)
- O<S>Se>Te (right trend)
- Please note that oxygen has less electron gain enthalpy than sulphur because due to small size of oxygen the incoming electron suffers repulsion therefore electron gain enthalpy is less negative as compared to sulphur.
This whole group is called chalcogens as they are ore forming.
For example:
- Al –bauxite ore
- Pb-galena
- Hg-cinnabar ore
- Zn-zinc blende and many more.
Elemental state of oxygen and sulphur
Oxygen exists as diatomic gas, whereas sulphur exists as S8 in solid form in a puckered ring like structure as shown below:
Sulphur (crown-like structure)
- In case of oxygen pπ-pπ bonding takes place but in sulphur no such bonding takes place .That is the reason oxygen exist as gas and sulphur exist as solid.
Chemical properties of group 16
Trends in chemical reactions:
- The order of reactivity of group 16 elements is:
O>S>Se>Te
That is oxygen is quite reactive and as we move down reactivity decreases.
- Oxygen in this group is quite reactive as the bond between oxygen atoms is quite strong and the energy required to break it is 493.3kJ/mol.
- Almost all the reactions of oxygen are exothermic, therefore, the reactions are called combustion reaction.
The most stable compounds of this group are: selenides and tellurides.
- Reaction with hydrogen: When group 16 elements combine with hydrogen they form hydrides with general formula H2
The hydrides formed are:
All of them have bent geometry with sp3 hybridization. The geometry shown by them is:
Structure of water
The properties of hydrides are:
- Bond angle: The bond angle decreases down the group. The order of decrease of angle is:
The reason behind this is that when size of central atom increases, lone pair will push closer to bond pair-bond pair. Due to this bond angle decreases.
- Basic character: It refers to ability of molecule to donate its lone pair. The order of increase in basic character is:
- Stability: The stability of hydrides depends upon the comparability of size. The order of stability of hydrides is:
Due to increase in size, bond length increase due to which bond dissociation energy decrease therefore stability decrease.
- Reducing nature: The order of reducing character of hydrides are:
Out of them in case of H2Te the bond strength is low therefore reducing character is lowest.
- Boiling point: The boiling point depends upon Vander wall force and this Vander wall force increases with increase in size.
The order of their boiling points for 15 group hydrides is:
In case of H2O and H2S, water has higher boiling point than hydrogen sulphide because of hydrogen bonding.
- Reaction with halogen: They react with halogen to form halides of formula EX4 , EX6 and EX2.
The order of stability oh halides are:
F>Cl>Br>I
The group 16 elements react with iodine to form tetra iodides.
- Oxygen only forms halides OF2 and O2F Out of them oxy difluoride is most stable halide.
- Sulphur forms SCl2 (sulphur dichloride), SF6 (sulphur hexafluoride) and SF4 (sulphur tetra fluoride).
- The geometry of SF4 is tetrahedral with hybridization sp3d and geometry of SF6 is trigonal bipyramidal with hybridization sp3d2 as shown:
Sulphur tetrafluoride Sulphur hexafluoride
Hexa halides are only formed by fluorine because as size increases, coordination number decreases, therefore others they will not form halides .
- Out of all halides sulphur hexahalide is most non reactive halide in this group because it has excess octet .So, no vacant d orbital is present moreover it is protected from all sides by fluorine .
3. OxO acids of sulphur
Some important oxo acids of sulphur are:
- Sulphurous acid: it is reducing in nature.
- It acts as oxidizing and reducing action.
- It bleaches the articles due to reduction.
- Its structure is given :
Sulphurous acid
(b)Sulphuric acid:
- It is called king of chemicals.
- It is used as an acid, oxidizing agent, dehydrating agent etc.
- It is highly viscous due to hydrogen bonding.
- It is also called as brown oil of vitrol.
- It is further concentrated and form rectified oil.
- It is prepared by contact process.
- Its structure is given below:
Sulphuric acid
(c)Thiosulphurous acid
- It contains double bond between S atoms.
- Its structure is given below:
Thiosulphurous acid
(d)Thiosulphuric acid:
- It contains one double bond S linkage between S atoms.
- Its structure is given below:
Thiosulphuric acid
(e)Dithionous acid
- It contains one S-S bond.
- Its structure is given:
Dithionous acid
(f)Pyrosulphuric acid
- It is known as oleum.
- It contains one S-S linkage.
- Sulphur dissolves in oleum to give clear brightly colored solution which contains polyatomic sulphur cations of general formula S42-.
- The color of solution depends upon the time of reaction and strength of oleum.
- The bright yellow color solution has S2-4 ions, deep blue solution S82+ and bright red solution has totally unexpected S2+19
- Its structure is given below:
Pyrosulphuric acid
(g)Dithionic acid:
- It contains one S-S bond.
- Its structure is given below:
Dithionic acid
(h)Peroxymonosulphuric acid:
- It contains one peroxo group, it is also known as Caro’s acid.
- It contains one peroxo group.
- In it the oxidation state of S is +6.
- Its structure is given below.
Peroxymonosulphuric acid
Di oxygen
Oxygen is 21% by volume of air.
It is prepared by Karl Wilhelm Scheele and the other reactions were done by Priestly.
Isotopes of oxygen: O16, O17, O18
Preparation of dioxygen
General method of preparation:
We get oxygen whenever we heat any metal oxide like:
In laboratory we prepare oxygen by:
By heating potassium chlorate
This reaction occurs on heating, in presence of MnO2 at 420k.
Industrial preparation: By electrolysis of water.
The silent electric discharge is passed so that the reaction do not becomes reversible.
H2 + O2 —> H2O
Properties of di oxygen
- It is colorless, odorless and tasteless gas.
- It is soluble in water.
- It is highly inflammable.
- Chemical properties.
- It is neutral to litmus solution.
- It is supporter of combustion.
- It oxidizes food and produce energy.
- Reaction with metals :
- Mg + O2 —> MgO
- Magnesium Oxide
- Al + O2 —> Al2O3
- Aluminium Oxide
It also reacts with some compounds:
SO2 + O2 —> SO3
NH3 + O2 —> NO + H2O
Uses of di oxygen
- It is supporter of life.
- It is used in oxy acetylene flame.
- Liquid oxygen is used as rocket fuel.
- The radioactive isotope of it is used as tracer for many chemical reactions.
- It is used to prepare synthesis gas.
Classification of oxides
We have different types of oxides
- Normal oxides
- Poly oxides
The poly oxides are of further different types:
- Peroxides: In this the O22- ion is present like H2O2
- Super oxides: In this the O2– ion is present like KO2
- Sub oxides: In this oxygen has valence less than -2 like N2
- Mixed oxides: In this oxygen has oxidation number in fraction like Fe3O4
On the basis of nature they are classified as:
- Acidic
- Basic
- Neutral
- Acidic oxides: That dissolve in water to give acids:
All non metallic are acidic in nature.
- Basic oxides: That dissolves in water to give bases.
The metal oxides are basic in nature.
- Amphoteric oxides: Metalloids give amphoteric oxides.
It behave as acid as well as base.
- Neutral oxides
They are very less in number. They are neither acidic nor basic
Example: laughing gas.
Ozone
It is represented as O3. It is found in upper atmosphere.
Preparation:
- It is prepared when ultraviolet rays. They react with oxygen of upper atmosphere and splits oxygen molecule in oxygen atoms. Then this oxygen atom combines with oxygen molecule to form ozone:
- 3O2 —> 2O3
- Oxygen Ozone
- The reaction is endothermic approx. 142.7kJ of heat is needed.
- For preparing pure ozone we use ozoniser, in which the electric spark is passed through oxygen gas and we get ozone.
Properties of ozone
Physical properties
- It is blue gas with pungent odour.
- It is heavier than air.
- It is slightly soluble in water.
- It is diamagnetic.
- The depletion of ozone is harmful for us.
Chemical properties
- Effect of heat: If we heat ozone t decompose to give oxygen molecule that is 2O3 —> 3O
- Oxidizing agent: It is stronger oxidizing agent than oxygen gas
Because it decomposes to form:
So both the oxygen form oxidizes the other.
Sulphuric acid
Its molecular formula is H2SO4.
Preparation of sulphuric acid
It is prepared from Contact’s process that is:
1st step: Preparation of sulphur dioxide:
S + O2 —> SO2
Sulphur Oxygen Sulphur dioxide
2nd step: Preparation of sulphur trioxide:
SO2 + O2 —-> SO3
Sulphur dioxide Oxygen Sulphur trioxide
3rd step: Reaction of sulphur trioxide with concentrated Sulphuric acid:
SO3 + H2SO4 –> H2S2O7
Sulphur Trioxide Sulphuric Acid Olleum
4TH step: Diluting this oleum:
H2S2O7 + H2O —> H2SO4(dilute)
Olleum Water Sulphuric Acid
This sulphuric acid formed is 99% pure.
Sulphuric acid
Its molecular formula is H2SO4.
Preparation of sulphuric acid
It is prepared from Contact’s process that is:
1st step: Preparation of sulphur dioxide:
S + O2 —> SO2
Sulphur Oxygen Sulphur dioxide
2nd step: Preparation of sulphur trioxide:
SO2 + O2 —-> SO3
Sulphur dioxide Oxygen Sulphur trioxide
3rd step: Reaction of sulphur trioxide with concentrated Sulphuric acid:
SO3 + H2SO4 –> H2S2O7
Sulphur Trioxide Sulphuric Acid Olleum
4TH step: Diluting this oleum:
H2S2O7 + H2O —> H2SO4(dilute)
Olleum Water Sulphuric Acid
This sulphuric acid formed is 99% pure.
It is highly reactive due to:
- Low volatility
- Strong acidic character
- Strong affinity for water
- Its ability to act as oxidizing agent
- Dissociation: Sulphuric acid is dibasic in nature
Because of formation of this bisulphate and sulphate ions it forms two types of salts bisulphate’s and bisulphites that is NaHSO4 and Na2SO4.
- Because of low volatility, it can be used for preparing volatile acids that is halogen acids.
- It behaves as dehydrating agent, that is, if it falls on skin it causes burning sensation.
In a same way it can convert sugar solution to black mass.
- It behaves as oxidizing agent.
It oxidizes many like Cu to Cu2+.
Uses of sulphuric acid
- It is used in making fertilizers.
- It is king of chemicals so have wide use in industries.
- It is dehydrating agent.
- It acts as an oxidizing agent.
Introduction
The members of group 17 are:
- Fluorine(F)
- Chlorine(Cl)
- Bromine(Br)
- Iodine (I)
- Astatine (At)
Physical properties of group 17
- 1. Electronic configuration-The general electronic configuration for this group is ns2np5
- Fluorine (F) [He]2s2,2p5
- Chlorine (Cl) [Ne]3s2,3p5
- Bromine(Br)[Ar]3d104s24p5
- Iodine (I)[Kr]4d10,5s2,5p4
- Astatine (At) [Xe]4f14,5d10,6s2,6p4
- Atomic size: It increases down the group as every time a new shell is added as we move down. If we compare size of group 17 elements with group 16 then we see that group 17 is smaller due to increased nuclear charge.
- Ionization energy: If we compare ionization energy of group 17 and group 16 we see that group 17 has higher energy than group 16 because of smaller size of group 17 elements.
- Electron gain enthalpy:
- Along period it is more negative because of attraction towards electron as of increased nuclear charge.
- Down the group electron gain enthalpy keeps on becoming less negative because nuclear charge decreases and size.
- So, the group 17 has highest electron gain enthalpy due to smallest size in periodic table.
- Please note that: Fluorine has less electron gain enthalpy than chlorine because due to small size of oxygen the incoming electron suffers repulsion therefore, electron gain enthalpy is less negative as compared to chlorine.
- Electro negativity: Group 17 elements are more electro negative than group 16 because of small size of group 17 elements. This group has highest electro negativity in whole periodic table.
Fluorine is most electronegative in periodic table. Down the group electronegativity decreases as the size increases.
- Melting point and boiling points: It increases down the group as size and mass increases the Vander wall force also increases therefore melting and boiling point increases.
- 7. Color: All halogens are colored like:
Fluorine: Dull yellow in color.
Chlorine: Greenish yellow.
Bromine: Reddish brown.
Iodine: Violet.
- Oxidation states: They show variable oxidation states like:
Fluorine: -1
Chlorine: -1, +1, +3, +7
Bromine : -1, +1, +3, +5, +7
Iodine: -1, +1, +3, +5, +7
- Higher oxidation states of halogens are used when they are combining with small size highly electronegative ions.
- All halogens are very reactive and reactivity decreases down the group.
- All act as Lewis acids as they accept electron.
- Fluorine is the strong oxidizing agent among all.
Anomalous behavior of Fluorine:
- Smallest size.
- Highest electronegativity.
- No vacant d orbital.
Low F-F bond dissociation energy.
Trends in chemical reactions
- Reaction with hydrogen: They form their respective halogen acids of formula HX that is :
HF (Hydrogen Fluroide) HCl (Hydrogen Chloride) HBr(Hydrogen Bromide)
HI (Hydrogen Iodide)
The properties of halogen acids are:
(a) All have linear structure with bond angle 180 degree.
(b) Out of all only HF is liquid otherwise all are gases.
HF is liquid due to hydrogen bonding they occur as associated molecules.
(c) Stability: The stability of hydrides depends upon the comparability of size. The order of stability of hydrides is:
HF > HCl > HBr > HI
Due to increase in size, bond length increase due to which bond dissociation energy decrease therefore stability decrease.
(d) Reducing nature: The order of reducing character of hydrides is:
HF < HCl < HBr < HI
All are Arrhenius acids. The HI has weakest bond therefore release of H is much easier.
(e) Boiling point: The boiling point depends upon Vander wall force and this Vander wall force increases with increase in size.
HF > HCl < HBr < HI
Due to hydrogen bonding HF has highest boiling point.
(f)Polarity: All halogen acids are polar in nature. Greater the size of anion more is the polarizibility more is the covalent character. Out of all, HF is highly polar.
- Reaction with oxygen: They react with oxygen to form their respective oxides.
Due to less electro negativity difference the oxides are un-stable.
The reason behind this trend is due to some thermodynamic reasons.
Chlorine gas (Cl2)
It was discovered by Scheele .The name of chlorine was given by Davy.
Preparation
Lab. Preparation:
- By heating pyrolusite ore that is MnO2 with HCl
(b)By heating potassium permanganate with KCl:
Industrial preparation:
Physical properties of chlorine gas:
- It is greenish yellow gas.
- It has strong pungent suffocating odor.
- It is poisonous gas.
- It is soluble in water and the solution so formed is chlorine water.
Chemical properties of chlorine gas
- Effect on litmus: Dry chlorine gas has no effect on litmus but the moist chlorine do have the effect, as it turns blue litmus red due to formation of HCl.
- Reaction with metals and non metals: It reacts with metals and non metals to form respective chlorides that is given below:
- It has great affinity for hydrogen to form HCl
H2 +Cl2 —>2HCl
- Reaction with ammonia
- Reaction with alkalis
- With dilute and cold sodium hydroxide
- With hot and concentrated NaOH:
With calcium hydroxide it forms bleaching powder:
- Reaction with hydrocarbons: It occurs in presence of light.
- Bleaching action of chlorine gas
It is due to nascent oxygen produced by moist chlorine:
Colored matter + [O] –> colorless matter
- Chlorine act as oxidizing agent: It is due to nascent oxygen.
For example:
- It oxidizes Fe2+ —> Fe3+
Uses of chlorine gas
- It is used for bleaching purpose.
- It is used in extraction of gold and silver.
- It is used in manufacture of dyes etc.
- It is used in preparation of poisonous gases like phosgene CoCl2, tear gas CCl3NO2
Hydrogen chloride
It was discovered by Glauber and the Davy was the one who said that it consist of H and Cl.
Preparation of hydrogen chloride
Preparation:
It is prepared by reacting sodium chloride with sulphuric acid and the following reaction occurs:
Properties of hydrogen chloride
- It is colorless gas with pungent smell.
- It can be easily liquefied.
- It freezes to white crystalline solid.
- It is highly soluble in water.
- It readily reacts with ammonia forming ammonium chloride:
- It forms aqua regia that is a mixture of HNO3 with HCl in ratio of 1:3 that helps in dissolving noble metals.
- It can decompose salts of weaker acids:
Uses of hydrogen chloride
- It is used in manufacturing of chlorine gas, ammonium chloride, etc.
- It is used for extracting glue from bones.
- It is used in making medicines.
- It is used in making aqua regia.
Oxy acids of halogens
The oxy acids of halogens are:
Fluorine
- HFO(+1) Hypofluorous acid
Chlorine
- HClO (+1) Hypochlorous acid
- HClO2 (+2)Chlorous acid
- HClO3(+3) Chloric acid
- HClO4(+4) Perchloric acid
Bromine
- HBrO(+1) Hypobromous acid
- HBrO3(+3) Bromic acid
- HBrO4(+4) Perbromic acid
Iodine
- HIO (+1) Hypoiodous acid
- HIO3 (+3) Iodic acid
- HIO4(+4) Periodic acid
Properties of oxy acids of halogens
Acidic strength :
- The acidic strength of these oxy acids having same oxidation number decreases with increase in size because the electro negativity decreases as we go down the group from chlorine to iodine.
- This is the reason that to withdraw electrons from oxygen atom towards itself decreases from chlorine to iodine.
- As a result the tendency to pull the electrons from hydrogen decreases and the release of hydrogen ion becomes difficult.
- The order of acidic strength is :
HClO<HClO2<HClO3<HClO4
- Oxidizing power and thermal stability :
- As the oxidation number increase the oxidation power decreases in order :
HClO>HClO2>HClO3>HClO4
- This is because the halogen oxygen bond becomes more covalent. As a result the thermal stability increases. Thus the hypohalites are stronger oxidizing agents than perhalates.
- With increase in oxidation number of halogen the thermal stability of both the acids and salts increases thus the stability of oxy acids are in order:
HClO<HClO2<HClO3<HClO4
- As the number of oxygen atoms increases in an ion, there is greater dispersal of negative charge therefore, greater is the stability of ion formed.
- The acidity of oxo- acids of different halogens which have same oxidation number decreases from chlorine to iodine. This is due to decrease in electro negativity with increase in size.
Inter-halogen compounds
The compound of on halogen with the other halogen is called inter halogen compounds.
They are formed due to large electro negativity and size difference between halogens.
Let’s take an example: Let’s assume two halogens A and B.
B is more electronegative than A then they will form four types of compounds:
- AB example: ClF (Chlorine monofluoride),BrF(Bromine monofluoride).
- AB3 example: ClF3 (Chlorine trifluoride), BrF3 (Bromine trifluoride).
- AB5 example: BrF5 (Bromine pentafluoride).
- AB7 example: IF7 (iodine heptafluoride).
The inter-halogen compounds are unstable and very reactive.
Properties:
- The larger halogen always serves as a central atom.
- The bonds formed are covalent.
- As the size difference decreases stability decreases and the polarity increases.
- Hydrolysis of these compounds always produces halide ion from smaller halogen and oxy halide from bigger halogen.
- They are strong oxidizing agents.
Structure of inter-halogen compounds:
- AB type – linear
- AB3 type – trigonal bi pyramidal
- AB5 type – square pyramidal
- AB7 type – pentagonal bipyramidal
Introduction
This group has noble gases or inert gases
The members of this group are:
Helium (He)
Neon (Ne)
Argon (Ar)
Krypton (Kr)
Xenon (Xe)
Radon (Rn)
Physical properties of group 18
- The electronic configuration for them are ns2np6
- Helium 1s 22s2
- Neon 1s2,2s2,2p6
- Argon 1s2,2s2,2p6,3s2,3p6
- krypton1s2,2s2,2p6,3s2,3p64s23d104p6
- xenon 1s2,2s2,2p6,3s2,3p64s23d104p64d105s25p6
- radon 1s2,2s2,2p6,3s2,3p64s23d104p64d105s25p64f145d106s26p6
2.Atomic size : : It increases down the group as every time a new shell is added as we move down. They actually have Vander wall radii.
- Ionization energy: They have highest ionization energy due to complete octet.
- 4. Electron gain Enthalpy: It is positive as they have complete octet so they have no attraction for incoming electron.
- Melting and boiling point: It is low due to weak force that exists that is Vander wall force.
Down the group size increases therefore Vander wall force also increases so as melting and boiling point increase.
- All noble gases are odorless and colorless and tasteless.
- All noble gases are sparingly soluble in water.
- All are inert gases as they have complete octet.
- All of them are monatomic.
Chemical properties of group 18
- According to the stable electronic configuration fact they have no compounds but the scientist Neil Bart let found that oxygen and xenon are very much similar.
Like both have almost same masses. Atomic radius of both is same.
- So, like oxygen combines with platinum fluoride in the same way xenon also combines:
Compounds of Xenon and fluorine
Xenon easily combines with fluorine to form xenon fluorides:
(a)XeF2
Preparation:
Xe + F2 —> XeF2
(Xenon) Fluroide XenonFluoride
2 : 1 (catalyst is nickel, 673k and 1 bar )
Structure:
Xenon difluoride
Reaction of it with water:
(b)XeF4 xenon tetrafluoride
Preparation:
The structure of xenon tetrafluoride is:
Xenon tetrafluoride
Reaction with water:
At normal temperature they are explosive in nature.
(c) XeF6 xenon hexafluoride
Preparation:
xenon hexafluoride
Reaction with water
(d) XeO3 xenon trioxide
Preparation:
The hybridization is: sp3.
The geometry is pyramidal.
The structure is:
xenon trioxide
(e) XeO4 (xenon tetra oxide)
The hybridization is sp3.
The geometry is tetrahedral.
The structure is given below:
xenon tetra oxide
(f) XeOF2 (xenon oxy fluoride)
The hybridization is sp3d.
The geometry is given below:
xenon oxy fluoride
(g) XeO2F2
The hybridization is sp3d2.
The geometry is given below:
Xenon dioxyfluoride
Uses of noble gases
- Helium is used in weather balloons.
- A mixture of helium and oxygen is used in cylinders by divers etc.
- Liquid helium is used maintain low temperature or we can say it is cryogenic liquid.
- Neon is used in sign board signals.
- Neon is used in filling up sodium vapor lamp.
- Neon is used in protecting electrical instruments like voltmeter.
- Argon is used in filling electric bulbs.
- Argon helps in providing inert atmosphere.
- Krypton is used in flash bulbs of high speed photography.
- Radon is used in treatment of cancer.
- Radon is used to treat metals.
NCERT Solutions for Class 12 Chemistry Chapter 7 The p-Block Elements
INTEXT Questions
Question 1. ( P Block Elements )
Why are pentahalides more covalent than trihalides?
Solution:
Higher the positive oxidation state of central atom, more will be its polarising power which, in turn, increases the covalent character of bond formed between the central atom and the other atom.
Question 2.
Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 element?
Solution:
Because BiH3 is the least stable among the hydrides of group 15.
Question 3.
Why is N2 less reactive at room temperature?
Solution:
Because of strong pπ – pπ overlap resulting into the triple bond. N = N due to which the bond dissociation energy of N2 is very high rendering it less reactive.
Question 4.
Mention the conditions required to maximise the yield of ammonia.
Solution:
N2 + 3H2 2NH3; ∆fH = -46.1 kj mol-1
In accordance with Le Chatelier’s principle high pressure and low temperature would favour formation of ammonia. The optimum conditions for the production of ammonia are a pressure of about 200 atm, a temperature of about 700K and use of a catalyst such as iron oxide with small amount of K2O and Al2,O3 as promoters.
Question 5.
How does ammonia react with a solution of Cu2+?
Solution:
NH3 in the form of solution reacts with Cu2+ to form a complex with deep blue colour.
Question 6.
What is the covalence of nitrogen in N2O5?
Solution:
From the structure of N2O5, it is evident that covalence of nitrogen is four.
Question 7.
Bond angle in PH4+ is higher than in PH3. Why?
Solution:
Both are sp3 hybridised. In PH4+ all the four orbitals are bonded whereas in PH3 there is a lone pair of electrons on P, which is responsible for lone pair-bond pair repulsion in PH3 reducing the bond angle to less than 109° 28′.
Question 8.
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2?
Solution:
When white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2, phosphine gas is liberated.
Question 9.
What happens when PCl5 is heated?
Solution:
On heating PCl5 sublimes and is converted to PCl3 on stronger heating.
Question 10.
Write a balanced equation for the hydrolytic reaction of PCl5 in heavy water.
Solution:
PCl5 + D2O → POCl3, + 2DCl
Question 11.
What is the basicity of H3PO4?
Solution:
Three P – OH groups are present in the molecule of H3PO4. Therefore, its basicity is three.
Question 12.
What happens when H3PO3 is heated?
Solution:
On heating phosphorous acid dispropotionates to give orthophosphoric acid and phosphine.
4H3PO3 → 3H3PO4 + PH3
Question 13.
List the important sources of sulphur.
Solution:
Combined sulphur exists as sulphates, such as gypsum, epsom, baryte and sulphides such as galena, zinc blende, copper pyrites, etc. Traces of sulphur occur as hydrogen sulphide in volcanoes. Few organic materials like eggs, proteins, garlic, onion, mustard, hair and wool contain sulphur. 0.03 – 0.1% sulphur is present in the earth’s crust.
Question 14.
Write the order of thermal stability of the hydrides of group 16 element.
Solution:
H2O > H2S > H2Se > H2Te
Question 15.
Why is H2O a liquid and H2S a gas?
Solution:
Because of small size and high electronegativity of oxygen, molecules of water are highly associated through hydrogen bonding resulting in its liquid state.
Question 16.
Which of the following does not react with oxygen directly? Zn,Ti, Pt, Fe.
Solution:
Platinum does not react with oxygen directly
Question 17.
Complete the following reactions :
- C2H4 + O2 →
- 4Al + 3 O2 →
Solution:
- C2H4 + 3O2 → 2CO2 + 2H2O
- 4Al + 3O2 → 2Al2O3
Question 18.
Why does O3 act as a powerful oxidising agent?
Solution:
Due to the ease with which ozone liberates nascent oxygen atoms, it acts as a powerful oxidising agent.
O3 → O2 + O
Question 19.
How is O3 estimated quantitatively?
Solution:
When ozone reacts with an excess of KI solution buffered with a borate buffer (pH = 9.2), iodine is liberated which can be titrated against standard solution of sodium thiosulphate. This is used as a method of estimation of ozone quantitatively.
Question 20.
What happens when sulphur dioxide is passed through an aqueous solution of Fe(lll) salt?
Solution:
When sulphur dioxide is passed through an aqueous solution of ferric ions, ferric ions are reduced to ferrous ions.
Question 21.
Comment on the nature of two S — O bonds formed in SO2 molecule. Are the two S — O bonds in this molecule equal?
Solution:
Both the S—O bonds are covalent and have equal strength due to resonating structures.
Question 22.
How is the presence of SO2 detected?
Solution:
Presence of SO2 is detected by bringing a paper dipped in acidified potassium dichromate near the gas. If the paper turns green, it shows the presence of SO2 gas.
Question 23.
Mention three areas in which H2SO4 plays an important role.
Solution:
- In manufacture of fertilisers.
- In manufacture of pigments, paints and dyestuff intermediates.
- In detergent industry
Question 24.
Write the conditions to maximise the yield of H2SO4 by Contact process.
Solution:
The key step in the manufacture of sulphuric acid is oxidation of SO2 to SO3 in presence of V2O5 catalyst.
The reaction is exothermic and reversible. Hence, low temperature and high pressure are the favourable conditions for maximum yield of SO3. In practice a pressure of 2 bar and temperature of 720 K is maintained.
Question 25.
Why is Ka2 « Ka1 for H2SO4 in water?
Solution:
H2SO4 is a very strong acid in water largely because of its first ionisation to H3O+ and HSO4– The ionisation of HSO4– to H3O+ and SO42- is very very small. That is why, Ka2« Ka1.
Question 26.
Considering the parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy, compare the oxidising power of F2 and Cl2.
Solution:
Fluorine is a better oxidising agent than chlorine because E°F2/F- is higher than E°Cl2/Cl- It is mainly due to low bond dissociation energy, high hydration energy and lower electron gain enthalpy, non-availability of d-orbitals in valence shell, that results in higher reduction potential of F2 than chlorine.
Question 27.
Give two examples to show the anomalous behaviour of fluorine.
Solution:
- Ionisation enthalpy, electro-negativity and electrode potential are higher for fluorine than the expected trends of other halogen.
- Fluorine does not show any positive oxidation state except in HOF.
Question 28. ( P Block Elements )
Sea is the greatest source of some halogens. Comment.
Solution:
Sea water contains chlorides, bromides and iodides of sodium, potassium, magnesium and calcium but sodium chloride being the maximum makes sea water saline. Various sea weeds contain upto 0.5% iodine.
Question 29. ( P Block Elements )
Give the reason for bleaching action of Cl2.
Solution:
Chlorine bleaches by oxidation Cl2 + H2O → HCl + HOCl → HCl + [O]
The nascent oxygen reacts with dye to make it colourless.
Question 30. ( P Block Elements )
Name two poisonous gases which can be prepared from chlorine gas.
Solution:
COCl2 (phosgene), CCl3NO2 (tear gas)
Question 31. ( P Block Elements )
Why is ICI more reactive than l2?
Solution:
In general, interhalogen compounds are more reactive than halogens due to weaker X-X’ bonding than X-X bond. Thus, ICI is more reactive than I2.
Question 32. ( P Block Elements )
Why is helium used in diving apparatus?
Solution:
A mixture of helium and oxygen does not cause pain due to very low solubility of helium in blood as compared to nitrogen.
Question 33. ( P Block Elements )
Balance the following equation :
XeF6 + H2O → XeO2F2 + 4HF
Solution:
Question 34. ( P Block Elements )
Why has it been difficult to study the chemistry of radon?
Solution:
Radon is radioactive with very short half-life which makes the study of chemistry of radon difficult.
NCERT Exercises
Question 1. ( P Block Elements )
Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Solution:
The valence shel 1 electronic configuration of group 15 elements is ns2np3. Due to half- filled p-orbitals, these elements have extra stability associated with them.
The common oxidation states of these elements are – 3, +3 and +5. The tendency to exhibit -3 oxidation state decreases down the group. The stability of +5 state decreases and that of +3 state increases down the group due to inert pair effect.
The size of group 15 elements increases down the group. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to presence of completely filled d and or f orbitals in heavier members.
Down the group, ionisation enthalpy decreases due to increase in atomic size. Due to stable half-filled configuration, they have much greater value than that of group 14 elements.
The electronegativity value, in general, decreases down the group with increasing atomic size. However, amongst the heavier elements, the difference is not that much pronounced.
Question 2. ( P Block Elements )
Why does the reactivity of nitrogen differ from phosphorus?
Solution:
Nitrogen has a unique ability to form pπ – pπ multiple bonds with itself and with other elements having small size and high electronegativity. Consequently, its bond enthalpy is very high and reactivity is less. Another factor which affects the chemistry of N is the absence of d-orbitals in its valence shell. As a result, not only the covalency of N is restricted to four, it can also not form dn – pn bonds. P on the other hand, does not form pn – pn bonds and hence it only forms a P – P single bond, which can easily be broken. Also, phosphorus has vacant d-orbitals and can form dn – dn bond with transition metals to form compounds which can act as ligands.
Question 3. ( P Block Elements )
Discuss the trends in chemical reactivity of group 15 elements.
Solution:
Nitrogen has very low reactivity due to unavailability of vacant d-orbital and high bond dissociation energy of N ≡ N bond.
(a) Hydrides : General formula for hydrides is MH3, e.g., NH3, PH3, ASH3, SbH3, BiH3. All these hydrides are covalent in nature and have pyramidal structure (sp3 hybridized).
(b) Halides – Elements of group 15 form two types of halides viz. trihalides and pentahalides. The halides are predominantly basic (Lewis bases) in nature and have lone pair of electrons (central atom is sp3 hybridized). The pentahalides are thermally less stable than the trihalides.
(c) Oxides – All the elements of this group form two types of oxides ie., M2O3 and M2O5 and are called trioxides and pentoxides
Question 4. ( P Block Elements )
Why does NH3 form hydrogen bond but PH3 does not?
Solution:
Hydrogen bond is formed between electronegative atom and hydrogen atom. Nitrogen is an electronegative atom and electronegativity decreases down the group so PH3 cannot form H – bond.
Question 5. ( P Block Elements )
How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved.
Solution:
In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
Small amounts of NO and HNO3 are also formed in this reaction. These impurities can be removed by passing the gas through aqueous sulphuric acid containing potassium dichromate. It can also be obtained by the thermal decomposition of ammonium dichromate.
Very pure nitrogen can be obtained by the thermal decomposition of sodium or barium azide.
Question 6. ( P Block Elements )
How is ammonia manufactured industrially?
Solution:
Industrially ammonia is manufactured by Haber’s process.
In accordance with Le-Chatelier’s principle, high pressure and low temperature would favour the formation of ammonia. The optimum conditions for production of ammonia are a pressure of 200 × 105 Pa (about 200 atm), a temperature of -700 K and the use of a catalyst such as iron-oxide with small amounts of K2O and Al2O3 to increase the rate of attainment of equilibrium.
Question 7. ( P Block Elements )
Illustrate how copper metal can give different products on reaction with HNO3.
Solution:
acid is a strong oxidising agent and attacks most metals. The products of oxidation depend upon the concentration of the acid, temperature and the nature of the material undergoing oxidation. Copper with . cold dil. HNO3 forms nitric oxide (NO).
Question 8. ( P Block Elements )
Give the resonating structures of NO2 and N2O5.
Solution:
Resonating structures
Question 9. ( P Block Elements )
The HNH angle value is higher than HPH, HAsH and HSbH angles. Why?
[Hint : Can be explained on the basis of sp3 hybridisation in NH3 and only s-p bonding between hydrogen and other elements of the group].
Solution:
The actual bond angles are
The decreased bond angle in other hydrides can be explained by the fact that the sp3 hybridisation becomes less and less distinct with increasing size of the central atom i.e., pure p-orbitals are utilised in M-H bonding.
Question 10. ( P Block Elements )
Why does R3P = O exist but R3 N = O does not (R = alkyl group)?
Solution:
In R3 N = O, covalency required is five. The maximum covalency of nitrogen is four as it does not possess d-orbitals in the valence shell i.e., it cannot extend its valency beyond four. On the other hand, other members have d-orbitals and can utilise these orbitals to show covalency of five or six e.g., R3P = O, PCl5, [SbF6]– etc. These can form dπ – pπ bonds.
Question 11. ( P Block Elements )
Explain why NH3 is basic while BiH3 is only feebly basic.
Solution:
The basic character decreases from NH3 to BiH3. The basic nature is due to the presence of lone pair of electrons on the central atom. NH3 is the strongest electron pair donor due to its small size as the electron density of the electron pair is concentrated over a small region. As the size increases the electron density gets diffused over a large region and hence the ability to donate the electron pair (basic nature) decreases.
Question 12. ( P Block Elements )
Nitrogen exists as diatomic molecule and phosphorus as P4. Why?
Solution:
Nitrogen is diatomic gaseous molecule at ordinary temperature due to its ability to form pπ – pπ multiple bonds. The molecule has one σ and two π – bonds. Phosphorus exists as discrete tetratomic tetrahedral molecules as these are not capable of forming multiple bonds due to repulsion between non-bonded electrons of the inner core.
Question 13. ( P Block Elements )
Write main differences between the properties of white phosphorus and red phosphorus.
Solution:
Structure of white and red phosphorus are given below :
Question 14. ( P Block Elements )
Why does nitrogen show catenation properties less than phosphorus?
Solution:
The single N-N bond is weaker than the single P-P bond because of high interelectronic repulsion of the non-bonding electrons, owing to the small bond length. As a result the catenation tendency is weaker in nitrogen.
Question 15. ( P Block Elements )
Give the disproportionation reaction of H3PO3.
Solution:
The acids in +3 oxidation state of phosphorus tend to disproportionate to higher and lower oxidation states, e.g., phosphorous acid on heating disproportionates to give orthophosphoric acid and phosphine.
4H3PO3 → 3H3PO4 + PH3
Question 16. ( P Block Elements )
Can PCl5 act as an oxidising as well as a reducing agent? Justify.
Solution:
The oxidation state of P in PCl5 is +5. As P has five electrons in its valence shell, it cannot increase its oxidation state beyond +5 by donating electrons, therefore, PCl5 cannot act as a reducing agent. However, it can decrease its oxidation number from +5 to +3 or some lower value, so, PCl5 acts as an
oxidising agent. For example, it oxidises Ag to AgCl, Sn to SnCl4.
Question 17. ( P Block Elements )
Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuration, oxidation state and hydride formation.
Solution:
All these elements have same ns2np4 (n = 2 to 6) valence shell electronic configuration and hence are justified to be placed in group 16 of the periodic table.
(ii) Oxidation states : They need two more electrons to form dinegative ions by acquiring the nearest noble gas configuration. So, the minimum oxidation state of these elements should be -2. Oxygen predominantly and sulphur to some extent being electronegative show an oxidation state of -2. Since these elements have six electrons in the valence shell, therefore, the maximum oxidation state they can show is, + 6. Other positive oxidation states shown by these elements are +2 and +4. Although, oxygen due to the absence of d-orbitals does not show oxidation states of +4 and +6. Thus, on the basis of minimum and maximum oxidation states, these elements are justified to be placed in the same group i.c., group 16 of the periodic table.
(iii) Formation of hydrides : All the elements complete their respective octets by sharing two of their valence electrons with ls-orbilal of hydrogen to form hydrides of the general formula EH2 i.c., H2O, H2S, H2Se, H2Te and H2Po. Therefore, on the basis of formation of hydrides of the general formula, EH2, these elements are justified to be placed in group 16 of the periodic table.
Question 18. ( P Block Elements )
Why is dioxygen a gas but sulphur a solid?
Solution:
Oxygen atom has the tendency to form multiple bonds (pπ – pπ interaction) with other oxygen atom on account of small size while this tendency is missing in sulphur
atom. The bond energy of oxygen-oxygen double bond (0 = 0) is quite large (about three times that of oxygen-oxygen single bond, O – O = 34.9 kcal mol-1) while sulphur-sulphur double bond (S = S) is not so large (less than double of sulphur-sulphur single bond, S – S = 63.8 kcal mob1). As a resul —O—O—O— chains are less stable as compared to O = O molecule while —S—S—S— chains are more stable than S = S molecule. Therefore, at room temperature, while oxygen exists as a diatomic gas molecule, sulphur exists as S8 solid.
Question 19. ( P Block Elements )
Knowing the electron gain enthalpy values for O → O– and O → O2- as -141 and 702 kJ mol-1 respectively, how can you account for the formation of large number of oxides having O2- species and not O– ?
Hint : Consider lattice energy factor in the formation of compound.
Solution:
This can be explained with the help of electronic configuration.
As O2- has most stable configuration amongst these. So, formation of O2- is much more easier. In solid state, large amount of energy (lattice enthalpy) is released to form divalent O2- ions. It is greater lattice enthalpy of O2- which compensates for the high energy required to remove the second electron.
Question 20. ( P Block Elements )
Which aerosols deplete ozone?
Solution:
CFC – Chlorofluorocarbon (freons) : These compounds commonly known as freons are introduced into the atmosphere from aerosol sprays and refrigerating equipments. They undergo photochemical decomposition and destroy ozone as shown by the following sequence of reactions.
Question 21. ( P Block Elements )
Describe the manufacture of H2SO4 by Contact process?
Solution:
Sulphuric acid is manufactured by the Contact process which involves three steps :
- burning of sulphur or sulphide ores in air to generate SO2.
- conversion of SO2 to SO3 by the reaction with oxygen in the presr nee of a catalyst (V2O5), and
- absorption of SO3 in H2SO4 to give oleum (H2S2O7).
A flow diagram for the manufacture of sulphuric acid is shown in the figure. The SO2 produced is purified by removing dust and other impurities such as arsenic compounds. The key step in the manufacture of H2SO4 is the catalytic oxidation of SO2 with O2 to give SO3 in the presence of V2O5 (catalyst).
The reaction is exothermic, reversible and the forward reaction leads to a decrease in volume. Therefore, low temperature and high pressure are the favourable conditions for maximum yield. But the temperature should not be very low otherwise rate of reaction will become slow.
In practice, the plant is operated at a pressure of 2 bar and a temperature of 720 K. The SO3 gas from the catalytic converter is absorbed in concentrated H2SO4 to produce oleum. Dilution of oleum with water give H2SO4 of the desired concentration. In the industry, two steps are carried out simultaneously to make the process a continuous one and also to reduce the cost.
Question 22. ( P Block Elements )
How is SO2 an air pollutant?
Solution:
SO2 present in atmosphere combines with water to give sulphuric acid.
2SO2 + O2 + 2H2O → 2H2SO4
This is called acid rain. Acid rain causes extensive damage to building and statues made of marble, limestone, etc. due to its reaction to give CaSO4.
CaCO3 + H2SO4 → CaSO4 + CO2 + H2O
This way SO2 acts as pollutant.
Question 23. ( P Block Elements )
Why are halogens strong oxidising agents?
Solution:
General electronic configuration of halogen is
It will easily accept one electron to fulfill its shell. This easily acceptance of electron makes halogens strong oxidising agents.
Question 24. ( P Block Elements )
Explain why fluorine forms only one oxoacid, HOF.
Solution:
Due to high electronegativity and absence of d orbitals, F does not form oxoacids such as HOFO, HOFO2 and HOFO3 in which the oxidation state of F is +3, +5 and +7. It just forms one oxoacid, i.e., HOF in which the oxidation state of F is + 1.
Question 25. ( P Block Elements )
Explain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
Solution:
Although O and Cl have about the same electronegativity, yet their atomic size (covalent radii) are much different: O = 66 pm and Cl = 99 pm. Thus, electron density per unit volume of oxygen atom is much higher than that of chlorine atom. Hence, oxygen forms hydrogen bonds while chlorine does not though both have approx, the same electronegativity.
Question 26. ( P Block Elements )
Write two uses of ClO2.
Solution:
ClO2 is a powerful oxidising agent. ClO2 is used as a bleaching agent for paper pulp and textiles and in water treatment.
Question 27. ( P Block Elements )
Why are halogens coloured?
Solution:
All halogens are coloured because of absorption of radiations in visible region which results in the excitation of outer electrons to higher level. By absorbing different quanta of radiation, they display different colours as
F2 → yellow
Cl2 → greenish yellow
Br2 → red
I2 → violet
Question 28. ( P Block Elements )
Write the reactions of F2 and Cl2 with water.
Solution:
Fluorine reacts vigorously with water and oxidises water to oxygen.
Chlorine dissolves in water to form chlorine water. It slowly reacts with the water to form a mixture of hydrochloric acid and hypochlorous acid.
Hypochlorous acid is very unstable. In presence of sunlight, it decomposes to give HCl and nascent oxygen.
This oxygen is responsible for oxidising and bleaching properties of chlorine.
Question 29. ( P Block Elements )
How can you prepare Cl2 from HCI and HCI from Cl2? Write reactions only.
Solution:
Preparation of chlorine by Deacon’s process : By oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 at 723 K
Question 30. ( P Block Elements )
What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Solution:
Question 31. ( P Block Elements )
What are the oxidation states of phosphorus in the following :
- H3PO3
- PCl3
- Ca3P2
- Na3PO4
- POF3?
Solution:
Question 32. ( P Block Elements )
Write balanced equations for the following :
- NaCI is heated with sulphuric acid in the presence of MnO2.
- Chlorine gas is passed into a solution of Nal in water.
Solution:
Question 33. ( P Block Elements )
How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Solution:
Xenon forms three binary fluorides, XeF2, XeF4 and XeF6 by the direct reaction of elements under appropriate experimental conditions.
Question 34. ( P Block Elements )
With what neutral molecule is ClO– isoelectronic? Is that molecule a Lewis base?
Solution:
Replace O– (9 electrons) in ClO– by F (9 electrons). The resulting neutral molecule is ClF. Since ClF can combine further with F to form ClF3, so, ClF is a Lewis base.
Question 35. ( P Block Elements )
How are XeO3 and XeOF4 prepared?
Solution:
Question 36. ( P Block Elements )
Arrange the following in the order of property indicated for each set :
- F2, Cl2, Br2, l2 – increasing bond dissociation enthalpy.
- HF, HCI, HBr, HI – increasing acid strength.
- NH3, PH3, ASH3, SbH3, BiH3 – increasing base strength.
Solution:
- l2 < Br2 < F2 < Cl2
- HF < HCI < HBr < HI
- NH3 > PH3 > AsH3 > SbH, > BiH3
Question 37. ( P Block Elements )
Which one of the following does not exist?
(i) XeOF4
(ii) NeF2
(iii) XeF2
(iv) XeF6
Solution:
(ii) Amongst all noble gases, only Xe (except KrF2) forms compounds.
Question 38. ( P Block Elements )
Give the formula and describe the structure of a noble gas species which is isostructural with :
- lCl–4
- IBr–2
- Br0–3
Solution:
(iii) Structure of BrO–3 : The central atom Br has seven electrons. Four of these electrons form two double bonds or coordinate bonds with two oxygen atoms while the fifth electron forms a single bond with O– ion. The remaining two electrons form one lone pair. Hence, in all there are three bond pairs and one lone pair around Br atom in BrO–3 Therefore, according to VSEPR theory, BrO–3 s should be pyramidal.
Here, BrO–3 has 26(7 + 3 × 6 + 1 = 26) valence electrons. A noble gas species having 26 valence electrons is XeO3 (8 + 3 × 6 = 26). Thus, like BrO–3, XeO3 is also pyramidal.
Question 39. ( P Block Elements )
Why do noble gases have comparatively large atomic sizes?
Solution:
The atomic radii of noble gases are by far the largest in their respective periods. This is due to the reason that noble gases have only van der Waals radii while others have cova lent radii, van der Waals radii, by definition are larger than covalent radii.
Question 40. ( P Block Elements )
List the uses of neon and argon gases.
Solution:
Uses of neon :
- Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes.
- Glow of different colours ‘neon signs’ can be produced by mixing neon with other gases.
- Neon bulbs and used in botanical gardens and in green houses.
Uses of argon:
- Argon is used mainly to provide an inert atmosphere in high temperature metallurgical processes such as arc welding of metals and alloys. In the laboratory, it is used for handling substance which are air sensitive.
- It is used in filling incandescent and fluorescent lamps where its presence retards the sublimation of the filament and thus increases the life of the lamp.
- It is also used in “neon signs” for obtaining lights of different colours.
Important Questions Class 12 Chemistry Chapter 7 – The p-Block Elements
1 Mark Questions
1. Write the elements of group 15?
Ans. The elements of group 15 are Nitrogen (N), Phosphorous (P) , Arsenic (As) , Antimony (Sb) and Bismuth (Bi).
2. Write chemical name & formulae of
a) Chile saltpetre
b) Indian saltpetre
Ans. (a) Chile saltpetre – Sodium nitrate –
(b) Indian saltpetre – Potasium nitrate –
3. What is special about the valence configuration of Group 15?
Ans. The valence configuration of 15 group is the s-orbital is completely filled and p-orbital is half filled . This half filled orbital gives extra stability to elements of this group.
4. The atomic radii increases considerably from N to P but very little increase is observed from As to Bi. why?
Ans. There is a considerable increase in size from N to P as expected but due to the presence of completely filled d- orbitals which have very poor shielding effects, the increases in size is very little from As to Bi.
5. Give reason for the following- the first ionization enthalpy of 15th group elements is higher than 16th group elements ?
Ans. Due to extra stability of half filled configuration, the first Ionisation enthalpy of 15th group elements is higher than 16th group configuration
6. How does metallic character vary down the 15 group & why?
Ans. The metallic character increases down the group due to decrease in ionization enthalpy and increase in size of atom.
7. What are the common oxidation states of this group?
Ans. The common oxidation states of the group are -3, +3 & +5.
8. What is the maximum covalence shown by N?
Ans. Nitrogen shows a maximum covalence of +4 because only four orbitals, one S and three P- orbitals are available for bonding in Nitrogen.
9. Bi (v) is a stronger oxidizing agent than Bi(III). Why?
Ans. Bi is more stable in +3 oxidation state in comparison to +5 due to inert pair effect therefore Bi (v) has a strong tendency to act as oxidizing agent.
10. Give an example showing disproportionation of oxidation state of nitrogen?
Ans.
Here Nitrogen is getting oxidized to a higher oxidation state as well as reduced to a lower oxidation state.
11. Complete and balance –

Ans. (i)
(ii)
(iii)
(iv)
(v)
(vi)
(vii)
(viii)
(ix)
(x)
12. What are the optimum conditions for maximum yield of ammonia?
Ans. The optimum conditions for the production of ammonia are Pa or 200 atm pressure, 700K temperature , and presence of catalyst such as iron oxide with and as promoters.
13. Ammonia is a Lewis base. Why?
Ans. Due to the presence of lone pairs on nitrogen atom of ammonia, it can donate electron pair and acts as a lewis base.
14. Ammonia has higher boiling and melting points than expected. Why?
Ans. In solid and liquid states, ammonia molecules are associated by intermolecular hydrogen bonding. There fore ammonia has higher boiling and melting points.
15. Give reasons-
a) Halogens have smallest atomic radii in their periods
b) The negative electron gain enthalpy of fluorine is less than that of chlorine.
c) All halogens are coloured.
d) The only possible oxidation state of fluorine -1.
e) Fluorine forms only one oxoacid.
f) The stability of hydrides follows the order HF>HCl>HBr>HI.
Ans. (a) Due to maximum effective nuclear charge, halogens have smallest atomic radii.
(b) Due to small size of fluorine atom, there are strong inter electronic repulsions in the small 2p orbital of fluorine and thus incoming electron does not experience much attraction and fluorine has less negative electron gain enthalpy than that of chlorine.
(c) Halogens absorb radiation in visible region which results in oxidation of electrons to higher energy level by absorbing different quanta of radiation, they show different colours.
(d) Since fluorine is most electronegative element and is short of only one electron for completing octet, it shows the only oxidation state of -1.
(e) Due to small size and high electro-negativity, Fluorine forms only one hypohalous acid.
(f) As the size of element increases down the group, the bond dissociation enthalpy for HX bond decreases making the bond weaker and weaker therefore the order of thermal stability is HI < HBr < HCl < HF.
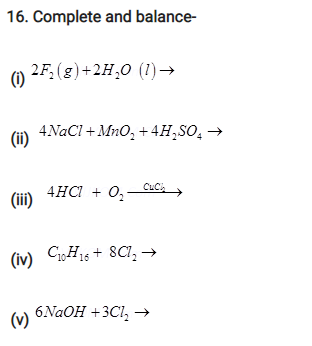
Ans. (i)
(ii)
(iii)
(iv)
(v)
17. Chlorine water on standing loses its yellow colour. Why?
Ans. On standing chlorine water forms HCl and Hypochlorous acid (HOCl) due to which it loses its colour.
18. Explain the bleaching action of chlorine?
Ans. The bleaching action of chlorine is due to its tendency to give nascent oxygen so that the substance gets oxidized.
Coloured substances + [O] colourless substance.
19. Write two uses of chlorine?
Ans. Chlorine is used for
a) Bleaching wood pulp, cotton and textiles
b) Manufacturing dyes, drugs, refrigerants etc.
c) Sterilizing drinking water.
20. Give reasons for the following?
(a) Nitrogen does not show catenation.
(b) exists but does not.
(c) The stability of Hydrides follows the order-
(d) PH3 is a weaker base than.
(e) Molecular nitrogen is chemically inert.
Ans. (a) Nitrogen being small in size has high electron density. Due to strong inter electronic repulsions, N-N single bond is weak & nitrogen does not undergo catenation.
(b) Due to absence of d-orbitals, nitrogen cannot expand its oxidation state to +5 and NCl5 does not exist whereas in P due to presence of empty 3d orbital +5 oxidation state is attained.
(c) As we move down the group 15, atomic radii increases making the bond of element with Hydrogen weaker this decreases the stability of hydrides of heavier elements. Therefore the order of stability is.
(d) As Phosphorous atom is larger than N- atom, the lone pair of electrons is distributed over a large surface area of P-atom than N-atom. Therefore the tendency of P to donate the lone pair of electrons is less.
(e) Molecular nitrogen is inert because N N bond energy is very high due to small size of N- atom and presence of multiple bond.
(e) Molecular nitrogen is inert because N N bond energy is very high due to small size of N- atom and presence of multiple bond.
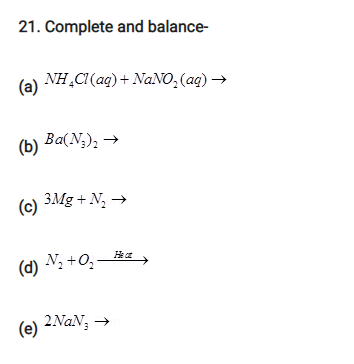
Ans. a.
b.
c.
d.
e.
22. What are the two isotopes of nitrogen? Write two uses of dinitrogen.
Ans. The two isotopes of nitrogen are .
23. Write the characteristics of pure Ozone?
Ans. Pure ozone is pale blue gas, dark blue liquid and violet black solid.
24. At what concentration ozone is harmful?
Ans. If the concentration of ozone increases above 100 ppm, breathing becomes uncomfortable resulting in headache and nausea and it becomes harmful.
25. Draw the resonating structures of ozone?
Ans.
26. Explain the oxidizing action of ozone?
Ans. Ozone has a very strong tendency to liberate nascent oxygen according to the equation:-
Therefore it acts as a strong oxidizing agent.
27. How is ozone estimated quantitatively?
Ans. When ozone reacts with an excess of potassium iodide solution buffered with a borate buffer (PH = 9.2) iodine is liberated. This iodine can be titrated against a standard solution of sodium thiosulphate to estimate the amount of ozone.
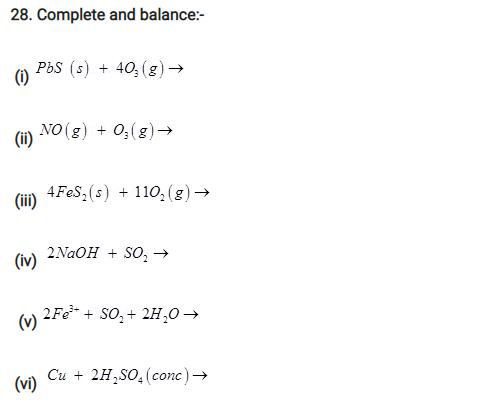
Ans. (i)
(ii)
(iii)
(iv)
(v)
(vi)
29. Give a test to detect the presence of gas?
Ans. When (g) is passed through a violet coloured acidified potassium permanganate solution, it gets decolourised.
30. Which aerosols and oxides deplete ozone layer
Ans. Freon used in aerosol sprays deplete the ozone layer. Nitrogen oxides emitted from the exhaust systems of supersonic jet aeroplanes deplete the ozone layer.
31. Write the members of 16 Group.
Ans. The elements of group 16 are: Oxygen(O), Sulphur (S), Selenium (Se), Tellurium (Te) and Polonium (Po)
32. Give the general electronic configuration of 16 Group.
Ans. The general electronic configuration of 16 group is ns2np4.
33. Discuss the geometry of.
Ans. In the hybridisation of sulphur is. The structure is trigonal bi-pyramidal in which one of the equatorial positions is occupied by a one pair of electrons. The geometry is called see-saw geometry.

Ans. (a)
(b)
(c )
(d)
(e)
(f)
35. Enlist some uses of dioxygen.
Ans. Oxygen is used-
(a) In oxyacetylene welding.
(b) In manufacture of steel.
(c) In hospitals and mountaineering as oxygen cylinders.
36. Write different isotopes of oxygen.
Ans. The isotopes of oxygen are .
37. Which of the following does not react with oxygen directly? Zn, Ti, Pt, Fe
Ans. Pt is a noble metal and does not react very easily. All other elements, Zn, Ti, Fe, are quite reactive. Hence, oxygen does not react with platinum (Pt) directly.
38. Why is ICl more reactive than ?
Ans. ICl is more reactive than because I-Cl bond in ICl is weaker than I-I bond in .
39. Balance the following equation:
Ans. Balanced equation:
40. How is ammonia manufactured industrially?
Ans. Ammonia is prepared on a large-scale by the Haber’s process.
41. Why does exist but does not (R = alkyl group)?
Ans. N(unlike P) lacks the d-orbital. This restricts nitrogen to expand its coordination number beyond four. Hence, does not exist.
42. Explain why fluorine forms only one oxoacid, HOF.
Ans. Fluorine forms only one oxoacid i.e., HOF because of its high electronegativity and small size.
43. Which one of the following does not exist?
(i) (ii)
(iii) (iv)
Ans. does not exist.
2 Marks Questions
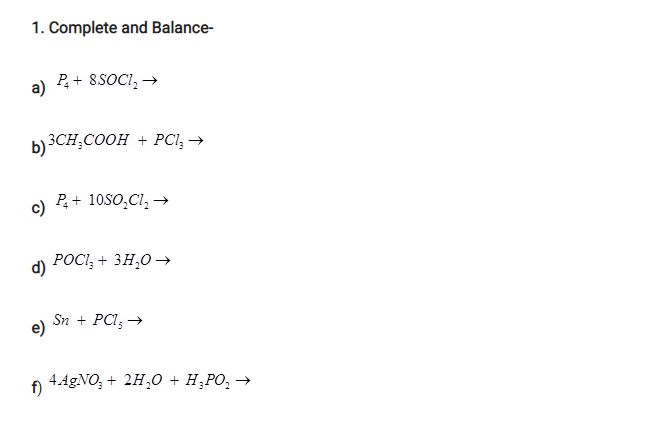
Ans. Complete and Balance-
a)
b)
c)
d)
e)
f)
2. All five bonds in are not equal. Give an equation in support of this statement.
Ans. When heated, loses a chlorine molecule this shows that two P- CL bonds are weaker and hence longer than others.

Ans. (i)
(ii)
(iii)
(iv)
(v)
(vi)
(vii)
4. Write two uses of dinitrogen.
Ans. Dinitrogen is used
(a) In the manufacture of ammonia.
(b) As a refrigerant to preserve biological material, food items.
(c) In cryosurgery.
5. Explain the chemistry behind brown ring test for detection of nitrate ions.
Ans. The brown ring test for nitrate ions depends on the ability of to reduce nitrates to nitric oxide, which reacts with Fe2+ to form a brown coloured complex.
6. Give the structures of
(i) sulphurous acid and
(ii) Peroxodisulphurous acid?
Ans. (i) Sulphurous acid
(ii) Peroxodisulphuric acid
7. Write the various steps for preparation of sulphuric acid by contact process?
Ans. Contact process for sulphuric acid:-
Step 1: Burning of sulphur in air to give.
Step 2: Conversion of by reacting it with oxygen in presence of.
Step 3: Absorption of in to give of oleum
Step 4: Dilution of oleum with water to get of desired concentration
8. Name different sulphates formed by sulphuric acid?
Ans. The two type of sulphates are –
(i) Normal sulphate eg.
(ii) acid sulphate eg..
9. Why are pentahalides more covalent than trihalides?
Ans. In pentahalides, the oxidation state is +5 and in trihalides, the oxidation state is +3. Since the metal ion with a high charge has more polarizing power, pentahalides are more covalent than trihalides.
10. Why is the strongest reducing agent amongst all the hydrides of Group 15 elements?
Ans. As we move down a group, the atomic size increases and the stability of the hydrides of group 15 elements decreases. Since the stability of hydrides decreases on moving from to , the reducing character of the hydrides increases on moving from to .
11. Why is less reactive at room temperature?
Ans. The two N atoms in are bonded to each other by very strong triple covalent bonds. The bond dissociation energy of this bond is very high. As a result, is less reactive at room temperature.
12. How does ammonia react with a solution of ?
Ans.acts as a Lewis base. It donates its electron pair and forms a linkage with metal ion.
13. What is the covalence of nitrogen in?
Ans.
From the structure of , it is evident that the covalence of nitrogen is 4.
14. What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of ?
Ans. White phosphorous dissolves in boiling NaOH solution (in a atmosphere) to give phosphine, .
15. Write a balanced equation for the hydrolytic reaction of in heavy water.
Ans. All the bonds that are present in are not similar. It has three equatorial and two axial bonds. The equatorial bonds are stronger are stronger than the axial ones. Therefore, when is heated strongly, it decomposes to form .
16. What happens when is heated ?
Ans.
Therefore, the net reaction can be written as
17. What is the basicity of ?
Ans.
Since there are three OH groups present in , its basicity is three i.e., it is a tribasic acid.
Concept Insight: Basicity is the Number of hydrogen that are replacable.
18. List the important sources of sulphur.
Ans. Sulphur mainly exists in combined form in the earth’s crust primarily as sulphates [gypsum (), Epsom salt , baryte ] and sulphides [(galena (PbS), zinc blends (ZnS), copper pyrites ].
19. Write the order of thermal stability of the hydrides of Group 16 elements.
Ans. The thermal stability of hydrides decreases on moving down the group. This is due to a decrease in the bond dissociation enthalpy (H-E) of hydrides on moving down the group.
Therefore,
20. Why is a liquid and a gas?
Ans. has oxygen as the central atom. Oxygen has smaller size and higher electronegativity as compared to sulphur. Therefore, there is extensive hydrogen bonding in , which is absent in . Molecules of are held together only by weak van der Waal’s forces of attraction.
Hence, exists as a liquid while as a gas.
21. Complete the following reactions:
(i)
(ii)
Ans. (i)
(ii)
Therefore, ozone acts as a powerful oxidising agent.
22. Why does act as a powerful oxidizing agent?
Ans. Ozone is not a very stable compound under normal conditions and decomposes readily on heating to give a molecule of oxygen and nascent oxygen. Nascent oxygen, being a free readical, is very reactive.
Therefore, ozone acts as a powerful oxidizing agent.
23. What happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt?
Ans. acts as a reducing agent when passed through an aqueous solution containing Fe(III) salt. It reduces Fe(III) to Fe(II) i.e., ferric ions to ferrous ions.
24. Give two examples to show the anomalous behaviour of fluorine.
Ans. Anomalous behaviour of fluorine
(i) It forms only one oxoacid as compared to other halogens that form a number of oxoacids.
(ii) Ionisation enthalpy, electronegativity, and electrode potential of fluorine are much higher than expected.
25. Sea is the greatest source of some halogens. Comment.
Ans. Sea water contains chlorides, bromides, and iodides of Na, K, Mg, and Ca. However, it primarily contains NaCl. The deposits of dried up sea beds contain sodium chloride and carnallite, . Marine life also contains iodine in their systems. For example, sea weeds contain upto 0.5% iodine as sodium iodide. Thus, sea is the greatest source of halogens.
26. Give the reason for bleaching action of .
Ans. When chlorine reacts with water, it produces nascent oxygen. This nascent oxygen then combines with the coloured substances present in the organic matter to oxide them into colourless substances.
Coloured substances + [O]’ Oxidized colourless substance
27. Name two poisonous gases which can be prepared from chlorine gas.
Ans. Two poisonous gases that can be prepared from chlorine gas are
(i) Phosgene
(ii) Mustard gas
28. Why has it been difficult to study the chemistry of radon?
Ans. It is difficult to study the chemistry of radon because it is a radioactive substance having a half-life of only 3.82 days. Also, compounds of radon such as have not been isolated. They have only been identified.
29. Why is helium used in diving apparatus?
Ans. Air contains a large amount of nitrogen and the solubility of gases in liquids increases with increase in pressure. When sea divers dive deep into the sea, large amount of nitrogen dissolves in their blood. When they come back to the surface, solubility of nitrogen decreases and it separates from the blood and forms small air bubbles. This leads to a dangerous medical condition called bends. Therefore, air in oxygen cylinders used for diving is diluted with helium gas. This is done as He is sparingly less soluble in blood.
30. Why does the reactivity of nitrogen differ from phosphorus?
Ans. Nitrogen is chemically less reactive. This is because of the high stability of its molecule,. In, the two nitrogen atoms form a triple bond. This triple bond has very high bond strength, which is very difficult to break. It is because of nitrogen’s small size that it is able to form bonds with itself. This property is not exhibited by atoms such as phosphorus. Thus, phosphorus is more reactive than nitrogen.
31. Why does form hydrogen bond but does not?
Ans. Nitrogen is highly electronegative as compared to phosphorus. This causes a greater attraction of electrons towards nitrogen in than towards phosphorus in . Hence, the extent of hydrogen bonding in is very less as compared to .
32. How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved.
Ans. An aqueous solution of ammonium chloride is treated with sodium nitrite.
NO and HNO3are produced in small amounts. These are impurities that can be removed on passing nitrogen gas through aqueous sulphuric acid, containing potassium dichromate.
33. Why does nitrogen show catenation properties less than phosphorus?
Ans. Catenation is much more common in phosphorous compounds than in nitrogen compounds. This is because of the relative weakness of the N-N single bond as compared to the P-P single bond. Since nitrogen atom is smaller, there is greater repulsion of electron density of two nitrogen atoms, thereby weakening the N-N single bond.
34. Give the disproportionation reaction of .
Ans. On heating, orthophosphorus acid disproportionates to give orthophosphoric acid and phosphine . The oxidation states of P in various species involved in the reaction are mentioned below.
35. Which aerosols deplete ozone?
Ans. Freons or chlorofluorocarbons (CFCs) are aerosols that accelerate the depletion of ozone. In the presence of ultraviolet radiations, molecules of CFCs break down to form chlorine-free radicals that combine with ozone to form oxygen.
36. Explain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
Ans. Both chlorine and oxygen have almost the same electronegativity values, but chlorine rarely forms hydrogen bonding. This is because in comparison to chlorine, oxygen has a smaller size and as a result, a higher electron density per unit volume.
37. Write two uses of .
Ans. Uses of :
(i) It is used for purifying water.
(ii) It is used as a bleaching agent.
38. Why are halogens coloured?
Ans. Almost all halogens are coloured. This is because halogens absorb radiations in the visible region. This results in the excitation of valence electrons to a higher energy region. Since the amount of energy required for excitation differs for each halogen, each halogen displays a different colour.
39. Write the reactions of and with water.
Ans.(i)
(ii)
40. How can you prepare from HCl and HCl from ? Write reactions only.
Ans.(i) can be prepared from HCl by Deacon’s process.
(ii) HCl can be prepared from Cl2on treating it with water.
41. What inspired N. Bartlett for carrying out reaction between Xe and?
Ans. Neil Bartlett initially carried out a reaction between oxygen and . This resulted in the formation of a red compound, .
Later, he realized that the first ionization energy of oxygen (1175 kJ/mol) and Xe (1170 kJ/mol) is almost the same. Thus, he tried to prepare a compound with Xe and . He was successful and a red-coloured compound, was formed.
42. Write balanced equations for the following:
(i) NaCl is heated with sulphuric acid in the presence of .
(ii) Chlorine gas is passed into a solution of NaI in water.
Ans. (i)
(ii)
43. With what neutral molecule is isoelectronic? Is that molecule a Lewis base?
Ans. is isoelectronic to ClF. Also, both species contain 26 electrons in all as shown.
Total electrons = 17 + 8 + 1 = 26
In ClF = 17 + 9 = 26
ClF acts like a Lewis base as it accepts electrons from F to form .
44. How are and prepared?
Ans. (i) can be prepared in two ways as shown.
(ii) can be prepared using .
45. Why do noble gases have comparatively large atomic sizes?
Ans. Noble gases do not form molecules. In case of noble gases, the atomic radii corresponds to van der Waal’s radii. On the other hand, the atomic radii of other elements correspond to their covalent radii. By definition, van der Waal’s radii are larger than covalent radii. It is for this reason that noble gases are very large in size as compared to other atoms belonging to the same period.
3 Marks Questions
1. Write three differences between white and red phosphorous.
Ans.
| White phosphorous | Red phosphorous |
| 1. It is translucent waxy solid | 1. It has iron grey Lustre. |
| 2. It is poisonous and glows in dark | 2. It is non poisonous and does not glow in dark. |
| 3. It is less stable and more reactive. | 3. It is more stable and less reactive. |
2. Why does dimerise?
Ans. contains odd number of valence electrons. It behaves as a typical odd molecule. On demerisation, it is converted to stable molecule with even number of electrons.
3. Mention the conditions required to maximise the yield of ammonia.
Ans. Ammonia is prepared using the Haber’s process. The yield of ammonia can be maximized under the following conditions:
(i) High pressure
(ii) A temperature of
(iii) Use of a catalyst such as iron oxide mixed with small amounts of and
4. What happens when H3PO3is heated?
Ans. ,on heating, undergoes disproportionation reaction to form and. The oxidation numbers of P in ,, and are +3, – 3, and +5 respectively. As the oxidation number of the same element is decreasing and increasing during a particular reaction, the reaction is a disproportionation reaction.
5. How is estimated quantitatively?
Ans. Quantitatively, ozone can be estimated with the help of potassium iodide. When ozone is made to react with potassium iodide solution buffered with a borate buffer (pH 9.2), iodine is liberated. This liberated iodine can be titrated against a standard solution of sodium thiosulphate using starch as an indicator. The reactions involved in the process are given below.
6. How is the presence of detected?
Ans. is a colourless and pungent smelling gas.
It can be detected with the help of potassium permanganate solution. When is passed through an acidified potassium permanganate solution, it decolonizes the solution as it reduces ions to ions.
7. Mention three areas in which plays an important role.
Ans. Sulphuric acid is an important industrial chemicaland is used for a lot of purposes. Some important uses of sulphuric acid are given below.
(i) It is used in fertilizer industry. It is used to make various fertilizers such as ammonium sulphate and calcium super phosphate.
(ii) It is used in the manufacture of pigments, paints, and detergents.
(iii) It is used in the manufacture of storage batteries.
8. Write the conditions to maximize the yield of by Contact process.
Ans. Manufacture of sulphuric acid by Contact process involves three steps.
1. Burning of ores to form
2. Conversion of to by the reaction of the former with
(is used in this process as a catalyst.)
3. Absorption of in to give oleum
The key step in this process is the second step. In this step, two moles of gaseous reactants combine to give one mole of gaseous product. Also, this reaction is exothermic. Thus, in accordance with Le Chatelier’s principle, to obtain the maximum amount of gas, temperature should be low and pressure should be high.
9. Why is for in water?
Ans.
It can be noticed that
This is because a neutral has a much higher tendency to lose a proton than the negatively charged . Thus, the former is a much stronger acid than the latter.
10. Considering the parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy, compare the oxidising power of and .
Ans. Fluorine is a much stronger oxidizing agent than chlorine. The oxidizing power depends on three factors.
1. Bond dissociation energy
2. Electron gain enthalpy
3. Hydration enthalpy
The electron gain enthalpy of chlorine is more negative than that of fluorine. However, the bond dissociation energy of fluorine is much lesser than that of chlorine. Also, because of its small size, the hydration energy of fluorine is much higher than that of chlorine. Therefore, the latter two factors more than compensate for the less negative electron gain enthalpy of fluorine. Thus, fluorine is a much stronger oxidizing agent than chlorine.
11. Illustrate how copper metal can give different products on reaction with .
Ans. Concentrated nitric acid is a strong oxidizing agent. It is used for oxidizing most metals. The products of oxidation depend on the concentration of the acid, temperature, and also on the material undergoing oxidation.
12. Give the resonating structures of and.
Ans.
(1)
(2)
13. The HNH angle value is higher than HPH, HAsH and HSbH angles. Why? [Hint: Can be explained on the basis of hybridisation in and only s-p bonding between hydrogen and other elements of the group].
Ans. Hydride
H-M-H angle
The above trend in the H-M-H bond angle can be explained on the basis of the electronegativity of the central atom. Since nitrogen is highly electronegative, there is high electron density around nitrogen. This causes greater repulsion between the electron pairs around nitrogen, resulting in maximum bond angle. We know that electronegativity decreases on moving down a group. Consequently, the repulsive interactions between the electron pairs decrease, thereby decreasing the H-M-H bond angle.
14. Explain why is basic while is only feebly basic.
Ans. is distinctly basic while is feebly basic.
Nitrogen has a small size due to which the lone pair of electrons is concentrated in a small region. This means that the charge density per unit volume is high. On moving down a group, the size of the central atom increases and the charge gets distributed over a large area decreasing the electron density. Hence, the electron donating capacity of group 15 element hydrides decreases on moving down the group.
15. Nitrogen exists as diatomic molecule and phosphorus as . Why?
Ans. Nitrogen owing to its small size has a tendency to form multiple bonds with itself. Nitrogen thus forms a very stable diatomic molecule, . On moving down a group, the tendency to form bonds decreases (because of the large size of heavier elements). Therefore, phosphorus (like other heavier metals) exists in the state.
16. Why is dioxygen a gas but sulphur a solid?
Ans. Oxygen is smaller in size as compared to sulphur. Due to its smaller size, it can effectively form bonds and form (O==O) molecule. Also, the intermolecular forces in oxygen are weak van der Wall’s, which cause it to exist as gas. On the other hand, sulphur does not form molecule but exists as a puckered structure held together by strong covalent bonds. Hence, it is a solid.
17. Knowing the electron gain enthalpy values for and as -141 and respectively, how can you account for the formation of a large number of oxides having species and not ?
(Hint: Consider lattice energy factor in the formation of compounds).
Ans. Stability of an ionic compound depends on its lattice energy. More the lattice energy of a compound, more stable it will be.
Lattice energy is directly proportional to the charge carried by an ion. When a metal combines with oxygen, the lattice energy of the oxide involving ion is much more than the oxide involving O-ion. Hence, the oxide having ions are more stable than oxides having . Hence, we can say that formation of is energetically more favourable than formation of .
18. Why are halogens strong oxidising agents?
Ans. The general electronic configuration of halogens is , where n = 2-6. Thus, halogens need only one more electron to complete their octet and to attain the stable noble gas configuration. Also, halogens are highly electronegative with low dissociation energies and high negative electron gain enthalpies. Therefore, they have a high tendency to gain an electron. Hence, they act as strong oxidizing agents.
19. How are xenon fluorides and obtained?
Ans. and are obtained by a direct reaction between Xe and . The condition under which the reaction is carried out determines the product.
20. Arrange the following in the order of property indicated for each set:
(i) increasing bond dissociation enthalpy.
(ii) HF, HCl, HBr, HI – increasing acid strength.
(iii) – increasing base strength.
Ans. (i) Bond dissociation energy usually decreases on moving down a group as the atomic size increases. However, the bond dissociation energy of is lower than that of and . This is due to the small atomic size of fluorine. Thus, the increasing order for bond dissociation energy among halogens is as follows:
(ii) HF < HCl < HBr < HI
The bond dissociation energy of H-X molecules where X = F, Cl, Br, I, decreases with an increase in the atomic size. Since H-I bond is the weakest, HI is the strongest acid.
(iii)
On moving from nitrogen to bismuth, the size of the atom increases while the electron density on the atom decreases. Thus, the basic strength decreases.
21. List the uses of Neon and argon gases.
Ans. Uses of neon gas:
(i) It is mixed with helium to protect electrical equipments from high voltage.
(ii) It is filled in discharge tubes with characteristic colours.
(iii) It is used in beacon lights.
Uses of Argon gas:
(i) Argon along with nitrogen is used in gas-filled electric lamps. This is because Ar is more inert than N.
(ii) It is usually used to provide an inert temperature in a high metallurgical process.
(iii) It is also used in laboratories to handle air-sensitive substances.
5 Marks Questions
1. Give reasons :-
(a) Oxygen molecule is diatomic where as sulphur molecule is polyatomic.
(b) The most common oxidation state of oxygen is -2.
(c) H2O is liquid whereas is gas at room temperature.
(d) The increasing order of acidic character in 16th group hydrides is.
(e) is exceptionally stable, does not exist.
Ans. (a) Oxygen being small in size forms effective and strong P-P bonds with other oxygen atom. Therefore oxygen molecule is diatomic and discrete whereas Sulphur due to its larger size, its orbitals cannot overlap effectively to form P-P bonds & completes valency by forming bonds with many sulphur atom. Therefore sulphur molecule is polyatomic solid.
(b) Since oxygen is highly electronegative, it has little tendency to give electrons. Therefore its most common oxidation state is -2.
(c) is liquid at room temperature due to presence of intermolecular Hydrogen bonding which is absent in.
(d) As we move down the group, the size of atom increases this make the bond of the element with hydrogen weak. Due to weaker bonds, the bond dissociation enthalpy decreases making the molecule more acidic. Therefore the order of acidic strength is.
(e) is exceptionally stable due to steric reasons. Hydrogen being electropositive or less electronegative than fluorine cannot make the s- electrons of sulphur to participate in bonding. Therefore does not exist.
2. Discuss the different types of oxides.
Ans. A binary compound of oxygen with another element is called oxide. Oxides can be simple or mixed. Simple oxides can be classified as acidic, basic Amphoteric or neutral.
An oxide that combines with water to give an acid is termed acidic oxide e.g., etc.
An oxide that combines with water to give a base is called basic oxide e.g., CaO, BaO etc.
An oxide that shows characteristics of both acids and bases is Amphoteric oxide e.g. .
An oxide that shows characteristic of neither acid nor base is called neutral oxide e.g. CO, NO and.
3. Bond angle in is higher than that in . Why?
Ans. In , P is hybridized. Three orbitals are involved in bonding with three hydrogen atoms and the fourth one contains a lone pair. As lone pair-bond pair repulsion is stronger than bond pair-bond pair repulsion, the tetrahedral shape associated with sp3bonding is changed to pyramidal. combines with a proton to form in which the lone pair is absent. Due to the absence of lone pair in, there is no lone pair-bond pair repulsion. Hence, the bond angle in is higher than the bond angle in .
Concept insite: the long pair-bond pair repulsion is more than bond pair-bond pair repulsion.
4. Comment on the nature of two S-O bonds formed in molecule. Are the two S-O bonds in this molecule equal?
Ans. The electronic configuration of S is .
During the formation of
, one electron from 3p orbital goes to the 3d orbital and S undergoes hybridization. Two of these orbitals form sigma bonds with two oxygen atoms and the third contains a lone pair. p-orbital and d-orbital contain an unpaired electron each. One of these electrons formsbond with one oxygen atom and the other forms bond with the other oxygen. This is the reason has a bent structure. Also, it is a resonance hybrid of structures I and II.
Both S-O bonds are equal in length (143 pm) and have a multiple bond character.
5. Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Ans. General trends in group15 elements
(i) Electronic configuration: All the elements in group 15 have 5 valence electrons. Their general electronic configuration is .
(ii) Oxidation states: All these elements have 5 valence electrons and require three more electrons to complete their octets. However, gaining electrons is very difficult as the nucleus will have to attract three more electrons. This can take place only with nitrogen as it is the smallest in size and the distance between the nucleus and the valence shell is relatively small. The remaining elements of this group show a formal oxidation state of -3 in their covalent compounds. In addition to the -3 state, N and P also show -1 and -2 oxidation states.
All the elements present in this group show +3 and +5 oxidation states. However, the stability of +5 oxidation state decreases down a group, whereas the stability of +3 oxidation state increases. This happens because of the inert pair effect.
(iii) Ionization energy and electronegativity
First ionization decreases on moving down a group. This is because of increasing atomic sizes. As we move down a group, electronegativity decreases, owing to an increase in size.
(iv) Atomic size: On moving down a group, the atomic size increases. This increase in the atomic size is attributed to an increase in the number of shells.
6. Discuss the trends in chemical reactivity of group 15 elements.
Ans. General trends in chemical properties of group – 15
(i) Reactivity towards hydrogen:
The elements of group 15 react with hydrogen to form hydrides of type , where E = N, P, As, Sb, or Bi. The stability of hydrides decreases on moving down from to .
(ii) Reactivity towards oxygen:
The elements of group 15 form two types of oxides: and , where E = N, P, As, Sb, or Bi. The oxide with the element in the higher oxidation state is more acidic than the other. However, the acidic character decreases on moving down a group.
(iii) Reactivity towards halogens: The group 15 elements react with halogens to form two series of salts: and . However, nitrogen does not form as it lacks the d-orbital. All trihalides (except ) are stable.
(iv) Reactivity towards metals: The group 15 elements react with metals to form binary compounds in which metals exhibit -3 oxidation states.
7. Write main differences between the properties of white phosphorus and red phosphorus.
Ans.
| White phosphorus | Red Phosphorus |
| It is a soft and waxy solid. It possesses a garlic smell. | It is a hard and crystalline solid, without any smell. |
| It is poisonous. | It is non-poisonous. |
| It is insoluble in water but soluble in carbon disulphide. | It is insoluble in both water and carbon disulphide. |
| It undergoes spontaneous combustion in air. | It is relatively less reactive. |
| In both solid and vapour states, it exists as a P4 molecule. | It exists as a chain of tetrahedral P4 units. |
8. Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuration, oxidation state and hydride formation.
Ans. The elements of group 16 are collectively called chalcogens.
(i) Elements of group 16 have six valence electrons each. The general electronic configuration of these elements is , where n varies from 2 to 6.
(ii) Oxidation state:
Asthese elements have six valence electrons , they should display an oxidation state of -2. However, only oxygen predominantly shows the oxidation state of -2 owing to its high electronegativity. It also exhibits the oxidation state of -1 , zero , and +2. However, the stability of the -2 oxidation state decreases on moving down a group due to a decrease in the electronegativity of the elements. The heavier elements of the group show an oxidation state of +2, +4, and +6 due to the availability of d-orbitals.
(iii) Formation of hydrides:
These elements form hydrides of formula , where E = O, S, Se, Te, PO. Oxygen and sulphur also form hydrides of type . These hydrides are quite volatile in nature.
9. Describe the manufacture of by contact process?
Ans. Sulphuric acid is manufactured by the contact process. It involves the following steps:
Step (i): Sulphur or sulphide ores are burnt in air to form .
Step (ii): By a reaction with oxygen, is converted into in the presence of as a catalyst.
Step (iii): produced is absorbed on to give (oleum).
This oleum is then diluted to obtain of the desired concentration.
In practice, the plant is operated at 2 bar (pressure) and 720 K (temperature). The sulphuric acid thus obtained is 96-98% pure.
10. How is an air pollutant?
Ans. Sulphur dioxide causes harm to the environment in many ways:
1. It combines with water vapour present in the atmosphere to form sulphuric acid. This causes acid rain. Acid rain damages soil, plants, and buildings, especially those made of marble.
2. Even in very low concentrations, causes irritation in the respiratory tract. It causes throat and eye irritation and can also affect the larynx to cause breathlessness.
3. It is extremely harmful to plants. Plants exposed to sulphur dioxide for a long time lose colour from their leaves. This condition is known as chlorosis. This happens because the formation of chlorophyll is affected by the presence of sulphur dioxide.
11. What are the oxidation states of phosphorus in the following:
(i) (ii) (iii)
(iv) (v) ?
Ans. Let the oxidation state of p be x.
(i)
3+x+3(–2) = 0
3 + x – 6 = 0
x – 3 = 0
x = +3
(ii)
x + 3(–1) =
x – 3 = 0
x = +3
(iii)
3(+2) + 2(x) = 0
6 + 2x = 0
2x = –6
x = –3
(iv)
3(+1) + x + 4(–2) = 0
3+x–8=0
x –5 = 0
x = +5
(v)
x + (–2) + 3(–1) = 0
x – 5 = 0
x = +5
12. Give the formula and describe the structure of a noble gas species which is isostructural with:
(i)
(ii)
(iii)
Ans. (i)is isoelectronic with and has square planar geometry.
(ii)is isoelectronic to and has a linear structure.
(iii)is isostructural to and has a pyramidal molecular structure.
Related Links
Class 9th
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]