www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Engineering post, Career Guidelines , English Speaking Trick , How to crack interview and lots more. ( Structure of Atom Notes and NCERT Solution )
Class 11th Chemistry Chapter 2 Structure of Atom NCERT Solutions
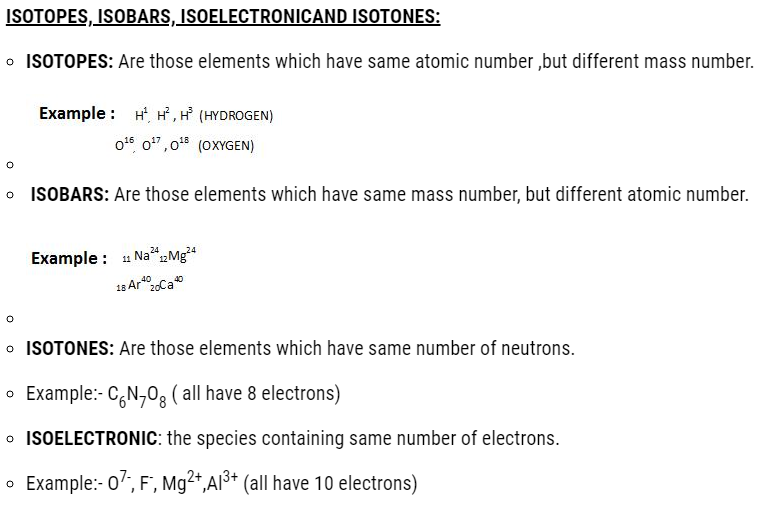
Class 11 Chemistry Chapter 2 Structure of Atom:
| Section Name | Topic Name |
| 2 | Structure of Atom |
| 2.1 | Discovery of Sub-atomic Particles |
| 2.2 | Atomic Models |
| 2.3 | Developments Leading to the Bohr’s Model of Atom |
| 2.4 | Bohr’s Model for Hydrogen Atom |
| 2.5 | Towards Quantum Mechanical Model of the Atom |
| 2.6 | Quantum Mechanical Model of Atom |
Class 11 Chemistry Structure of Atom
Introduction
As we have already studied about Daltons Atomic Theory of Matter, and later it was concluded that Atom is made up of 3 sub -atomic particles. The particles are Electron, Proton and Neutron. In this chapter ,we will study about different attempts made to explain atomic models .The phenomenon that leads to development of dual nature of Light and Matter .Also, we will deal with the methods to be followed while writing electronic configurations, and much more . Let us see that first how these fundamental particles were discovered, and how they are arranged in structure of Atom.
DISCOVERY OF ELECTRON:
The Electron was discovered by J.J Thomson by conducting a Cathode ray tube experiment.
For the experiment he used Crooke’s tube, which was 60cm long glass tube and had a small tube attached. To this small tube vacuum pump was attached, it also had two metal plates which were connected to battery by wires.

When current was passed under same conditions it also started glowing green. This confirmed that under those conditions some rays were emitted through cathode, and were travelling towards anode. Those rays were called as cathode rays and found to consist of negatively charged particles called electron.
Properties of cathode rays
They are found to travel in straight line. This property was concluded by performing an activity where the object was placed in their path. When they strike it ,they casted the shadow of the object as shown below:
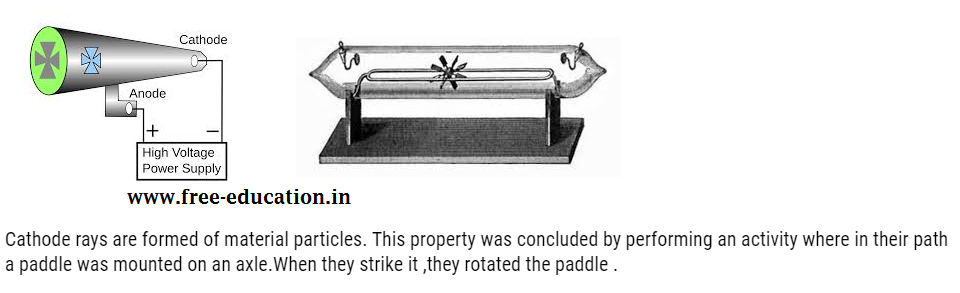

From the above properties and experiment it was concluded that cathode rays are made up of negatively charged particles called electron.
Further experiments were carried out in order to know the charge and mass of electron. It was found to be:
- Charge :1.6 x 10-19C
- Mass 9.1 x 10-31kg
We can define electron as:
- “A fundamental particle that is negatively charge of magnitude 1.6 x 10-19C and mass equal to 9.1 x 10-31
DISCOVERY OF PROTON:
IT WAS DONE BY E. GOLDSTEIN
- In 1836 the same experiment was carried out where the same conditions were provided. The gas at low pressure was taken and current was passed.
- The high voltage between the electrodes was passed. It was found that when cathode rays passed through the gas, they ionized the gas by taking electrons along, leaving behind positively ionized gas particles.
- These particles were travelling towards cathode.These particles were found to form the beam of rays of positively charged particles and were called as canal rays.
- These rays consist of positively charged particles called a proton.
Properties of anode rays
- They also travel in straight line.
- They are also made up of particles.
- They carry positive charge.
- Their ratio of charge to mass was different for different gas that was taken in tube.
- The mass of particles were found to be different for different gases. It was nearly equal to the mass of atom.
When hydrogen gas was taken in the tube its mass was found to be minimum. As we know, Hydrogen atom is the lightest one and its charge to be same as Electron. It is 1.6×10-19C and its e/m ratio taken as standard that is 1.67x 10-24g.
Proton is defined as a
“fundamental particle which carries one-unit positive charge and mass nearly same as hydrogen atom.”
DISCOVERY OF NEUTRON:
It was discovered by Chadwick. In which he bombarded some light elements with fast moving alpha particles. He found, that some new particles were emitted which carried no charge and had mass equal to that of proton.These neutral particles were named as neutron.
So, neutron is defined as:
“the fundamental particle which carries no charge and mass equal to that of hydrogen atom.”
ATOMIC MODELS OF ATOM
J.J THOMSON PLUM PUDDING MODEL
In 1904, the attempt to explain structure of Atom was done by J.J Thomson.





He said,
- Atom is electrically neutral.
- Nucleus is in center.
- In which protons & neutrons are present.
- Outside nucleus electrons revolve like planets revolve around sun.
He said, atom is made of two parts: nucleus and extra nuclear part .
In Nucleus, positive charge is present and in extra nuclear part electrons are present as shown in figure:
Proton -> (+)vely charged
Electron -> (-)vely charged
Neutron -> No charge
Limitation : he failed to explain the stability of an atom”.
According To Electromagnetic theory, a charged particle revolving in circular path continuously emits energy and shorten its path.

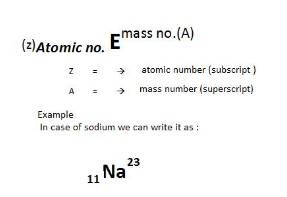

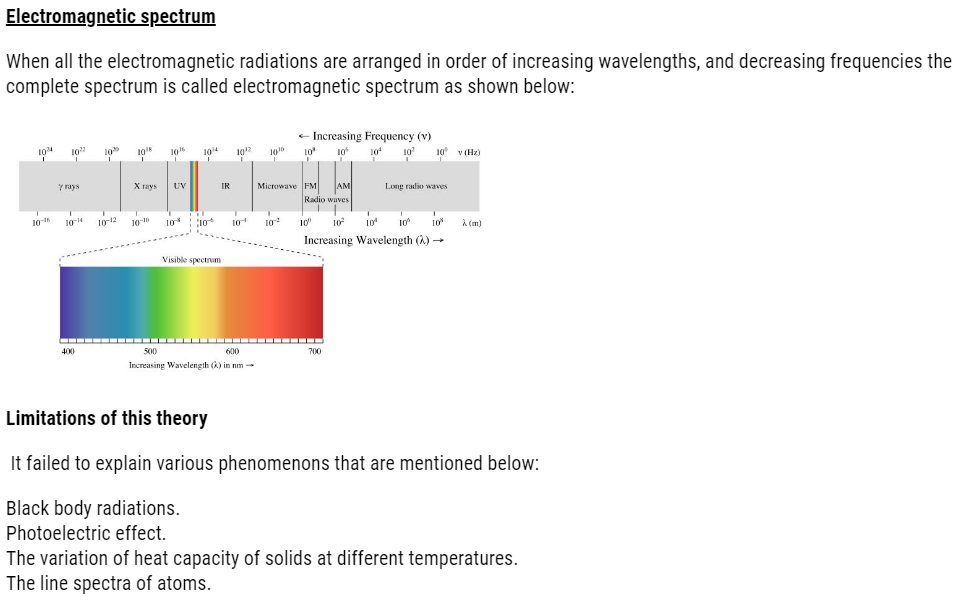
DUAL NATURE OF LIGHT: DEVELOPMENT LEADING TO BOHR’S MODEL ( Structure of Atom )
There were so many factors, phenomenon that lead to the failure of Rutherford model:
- Dual nature of matter
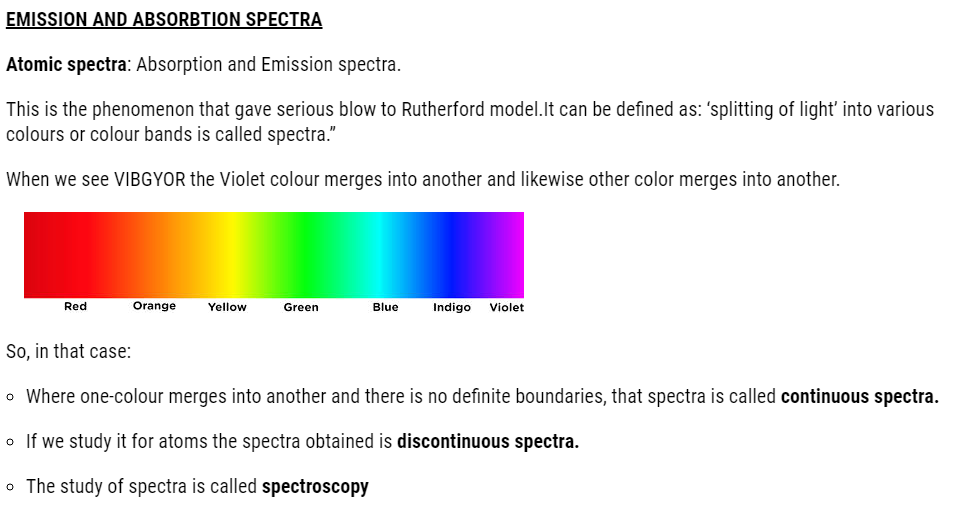
- Atomic spectra
But before studying Bohr model, we need to learn electromagnetic radiations.
Newton said the corpuscles of light possess wave nature of light.
It was able to explain reflection, refraction etc. But failed to explain phenomenon of interface or diffraction. So, particle nature of light was considered.
Let us study about dual nature. It is electromagnetic theory, which was given by Huygens.This proved wave like character of
Light and tells us about wave motion. Wave motion is like if you throw stones, you will see a ripple that is wave.
Electromagnetic theory: According to this theory
- Energy is emitted or absorbed continuously in the form of radiant energy.
- These radiations consist of electric and magnetic field acting perpendicular to the direction of propagation of wave.
- These waves can travel through medium as well as through vacuum.
- The radiations travel with speed =3×108m/sec.

- Frequency: number of waves produced in one second.
- Units Used:- sec-1, Hertz
- Velocity of light: It is distance travelled with respect to time in any direction.
- Units Used:- m/sec
- Amplitude: maximum displacement of particle from its mean position.
- Units Used:- m or cm
- Wave number: number of waves in 1cm length.
Black body radiation and Photoelectric effect ( Structure of Atom )
Particle nature of Electromagnetic radiations :There were two important phenomenon that couldn’t be explained by considering Light with wave character:
The phenomenon is:
- Black body radiation
- Photoelectric effect
Lets first study about the nature of these phenomenon:
- Black body radiation: Black body is defined as perfect emitter and absorber of light.
For example, whenever we heat an Iron ball like objects, on heating they become first Red, then Orange, then Yellow and at very high temperature they become White.

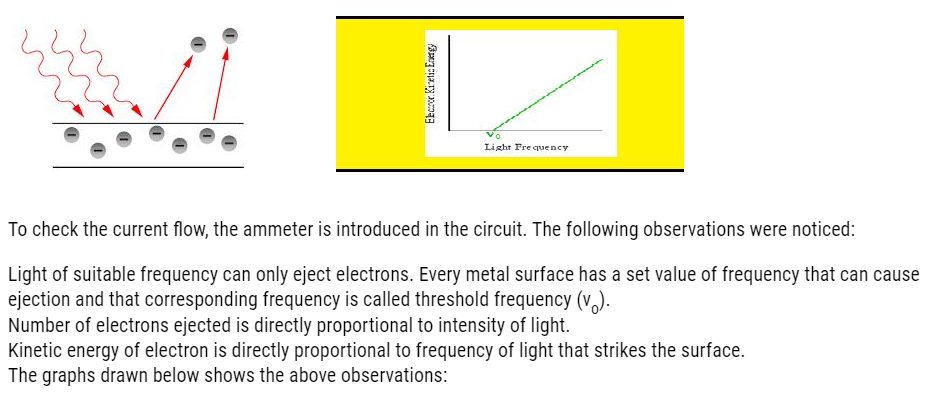
- Photoelectric effect
It is the ejection of electrons from metal surface when light of suitable wavelength strikes the metal surface.
The apparatus set to demonstrate this effect is given below:


Planck’s quantum theory:
According to this theory:
- Energy emitted or absorbed is not continuous, but is in the form of packets called quanta .In terms of light it is called as photon.
- Each photon carries an energy which is directly proportional to the frequency of wavelength i.e. E depends upon v (nu).
- Or E=hv (where v is frequency)
- Value of h =6.634 x 10-34Jsec
- Energy associated with no of packets is given by:
E=nhv (where n is an integral multiple)
This formula can also be written as:
E = (nhc)/ (λ)
(Because we know frequency=speed of light/wavelength)
V = (c/ λ)
EXPLANATION OF BLACK BODY AND PHOTOELECTRIC EFFECT ON THE BASIS OF QUANTUM THEORY.
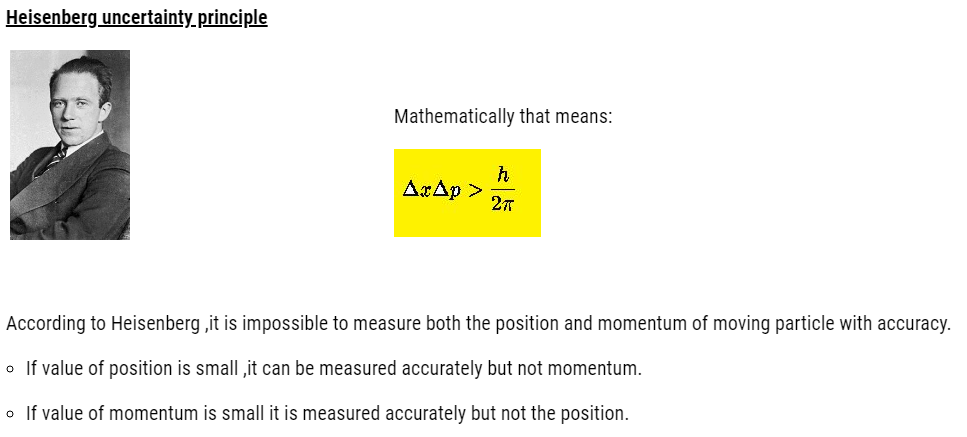
For the ejection, minimum frequency is required called threshold frequency .Let’s say light that falls, has energy equal to hv .Out of this h , a amount of h is used as binding energy and rest is given to electron as kinetic energy.
Mathematically




BOHR’S THEORY

EXPLANATION OF HYDROGEN SPECTRA
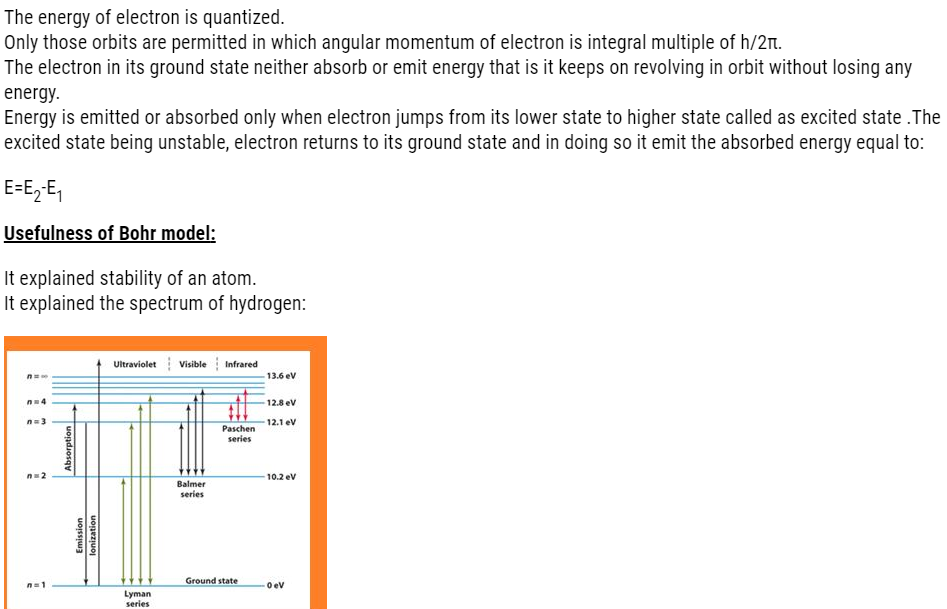
According to Bohr,
- When energy is supplied to atoms of hydrogen, the electron from lower energy gets excited to higher energy level.
- The excited state being unstable, it jumps back to its original state that is ground state.
- Some electrons move to their ground state in one jump, some in multiple jumps. Each jump corresponds to line in a spectrum.
- As we know the gas in tube consists of many hydrogen atoms.
- Therefore, each electron on getting energy gets excited.
- On returning to the ground state, they either move in single jump or multiple jump.
- This is the reason that we get so many lines in different regions in hydrogen spectrum.
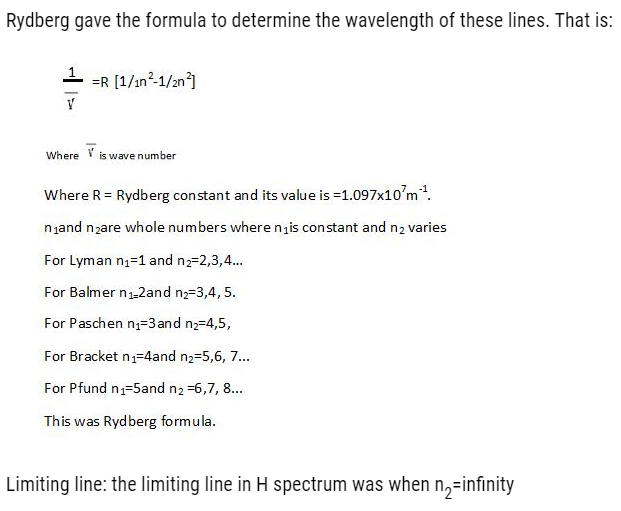
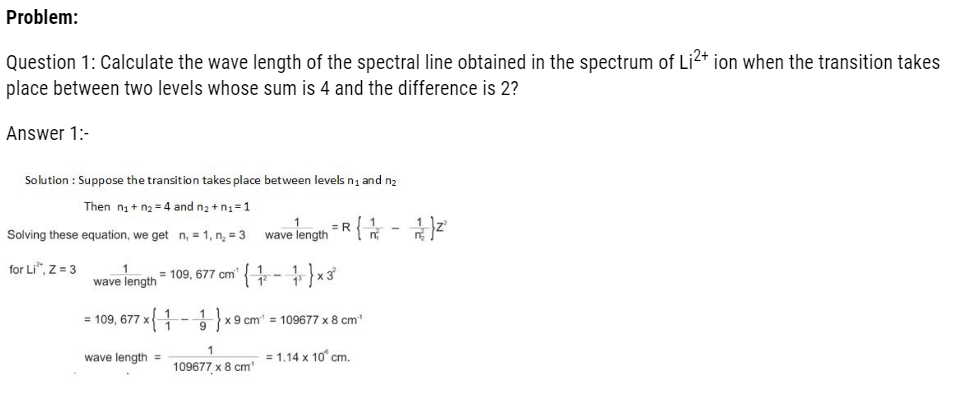
The wavelength emitted by them can be calculated as:





Significance
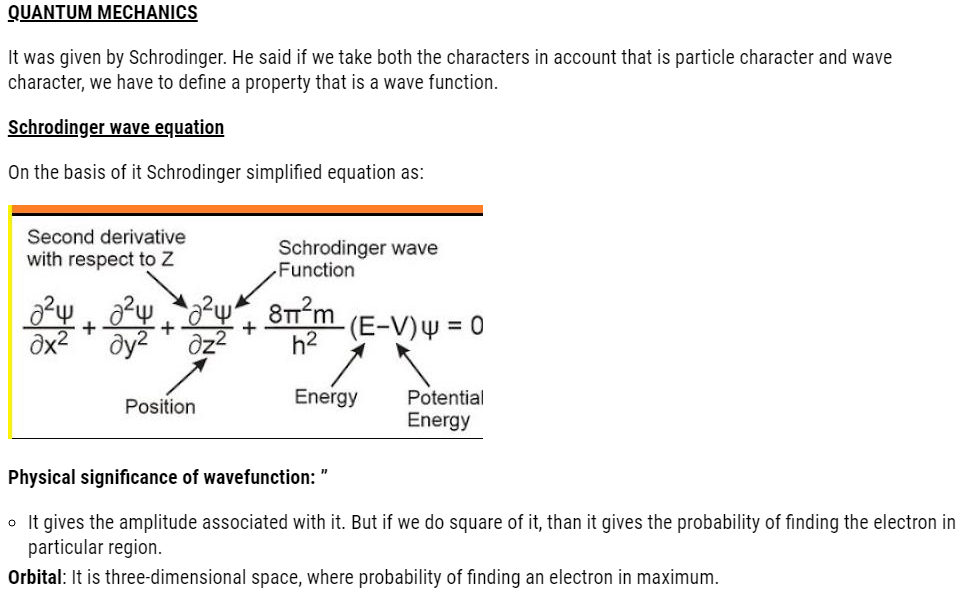
This holds good only for microscopic particles, as energy of photon is not enough to change the position and velocity of bigger bodies. So, in our daily routine it has no significance.

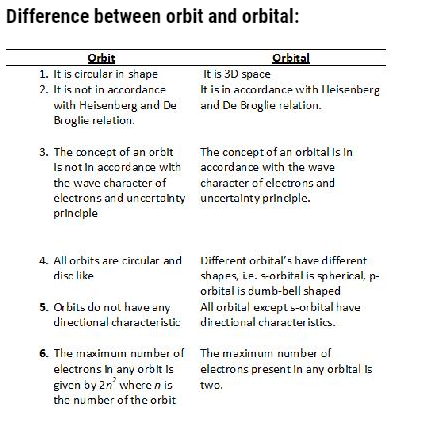
Orbital: They are of four types
- s orbital: Spherical in shape, non-directional. It has only 1 orbital therefore, can accommodate only 2 electrons.
- P-orbital: dumb-bell shaped and directional. It has 3 orbital (px, py, pz). It can accommodate maximum of 6 electrons.
- d-orbital: It has double dumbbell, directional. It has 5 orbital (dxy,dyz,dzx,dx2-y2,dz2).It can accommodate maximum of 10 electrons
- F-orbital: It has diffused shape. It has 7 orbital therefore, can accommodate maximum of 14 electrons.
Quantum numbers
They are set of 4 numbers, which give complete information about the address of electron.
There are 4 types of quantum numbers:
- Principal quantum number.
- Azimuthal quantum number.
- Magnetic quantum number.
- Spin quantum number.
Principal quantum number:
- It is represented as ‘n’.
- It was given by Bohr.
- It represents the orbit where electron is going to be present.
Uses:
- It gives number of electron in orbit by formula 2 n2.
- It gives angular momentum of electron.
- It gives energy of electron.
- It gives radius of orbit.
- Azimuthal quantum number:
- It gives information about sub shell of an atom.
- It is represented as ‘l’.
- It was introduced by Somerfield.
- It always has value (n-1).
Example: if n=1 ,l=0
If n=2,l=0,1
If n=3,l=0,1,2
- Magnetic quantum number:
- It describes the behavior of electron in magnetic field.
- It is represented as ‘m’.
- It was given by land.
- Its value is equal to –l,0, +l
For example: if n=1, l=0, m=0 that is only one orbital
If n=2, l=0,1, m=-1,0, +1 that is three orbitals
- Spin quantum number:
- It gives the info about spinning of electron about its axis i.e. clockwise or anticlockwise
- It is denoted by ‘s’.
- Its value is either =+1/2, -1/2
Problem: Write down the quantum numbers n , l and m for the
Following orbitals:
- 3d x2 – y2
- 4d z2
Answer :-
n=3, l=2, m=+2
N = 4, l=2, m=0
Pauli’s exclusion Principle
According to it: “no two electrons can have the same set of all four quantum numbers.”
Or, it states that an orbital can have maximum of two electrons and that must be of opposite spin. Due to this, it was concluded that an orbital can have maximum of two electrons which can have all 3-quantum number same but the spin will be definitely different.
Shapes of atomic orbitals:
- s –orbital
Its shape is spherical.For 1s the probability of finding electron is maximum near nucleus and it decreases as we move away from nucleus.
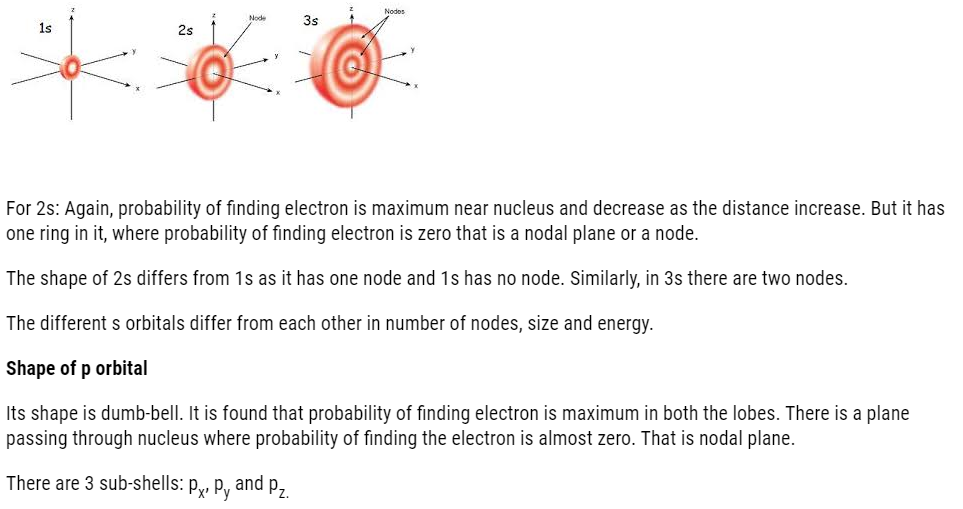
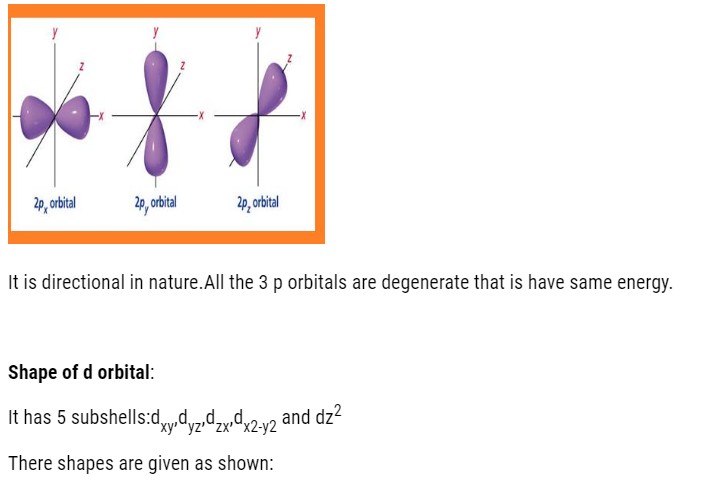
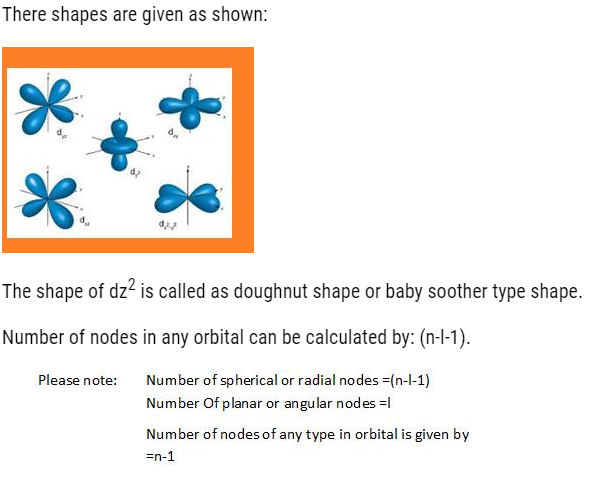

1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p…
To know the energy of orbital with lower energy the n+l rule is followed:
According to it:
- The lower the value of n+l for an orbital, the lower is its energy and is filled first.
For example: Out of 2s and 2p ,2s is filled first as n+l for 2s, is 2+0=2 and for 2p it is 2+1=3. Therefore, 2s is filled first.
- If two orbitals have same value of n+l , than the orbital with lower value of n is filled first.
For example: Out of 3p and 4s, n+l for both is 4 . Therefore, 3p is filled first as it possess lesser value of n.
- Pauli’s exclusion principle: According to it, an orbital can accommodate maximum of two electros and that must be of opposite spin.
For example: If orbital has s2, then the orbital has the arrangement as given below.

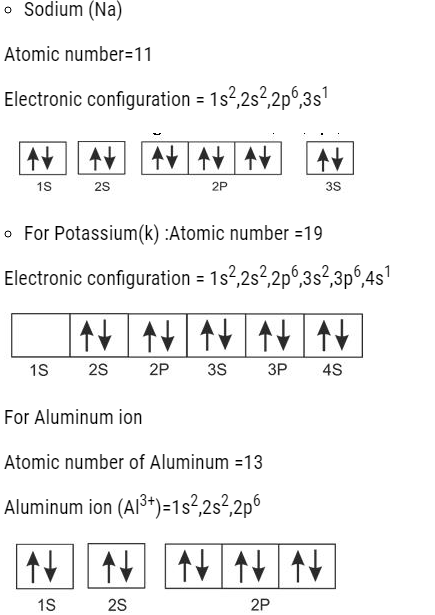
EXCEPTIONAL ELECTRONIC CONFIGURATION OF CHROMIUM AND COPPER
For Copper (Cu)= [Ar]18,4s23d9
But actually, it has : [Ar]18,4s13d10
Similarly, for Chromium it should be: [Ar]18,4s23d4
But in actual it is:=[Ar]18,4s13d5
The reason behind is:
- Half-filled and fully filled orbital are more stable
If configuration is 4s2 3d9 , then the d orbital is not fully filled. If configuration is 4s1 3d10 , then the d orbital is completely filled. That means it becomes more stable.
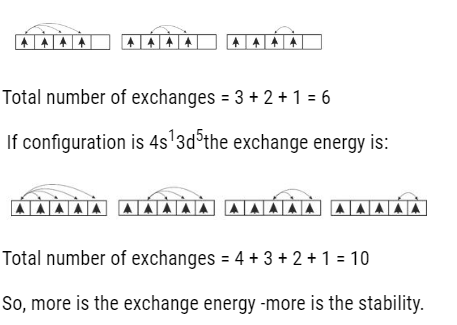
- The more is the exchange energy more stable is the orbital as shown below:
If configuration was 4s23d4 the exchange energy is:
Related link you must like:-
Study material for Competition Exam
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]