www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Technical Courses, Engineering post, Career Guidelines , English Speaking Trick , How to crack interview and lots more.( matter in our surroundings )
- Exercise-1.1-1.2 Page: 3
- Exercise-1.3 Page: 6
- Exercise-1.4 Page: 9
- Exercise-1.5 Page
- Exercise Page: 12 ( matter in our surroundings )
- Most Important Questions on Chapter 1: Matter in Our Surroundings
Matter In Our Surroundings Class 9 Notes – Chapter One
The topics and Subtopics from NCERT Solutions Class 9 Science Chapter 1- Matter in Our Surroundings are given below:
- Physical nature of matter
- Matter is Made Up of Particles
- How Small Are These Particles Of Matter?
- Characteristic of particles of matter
- Particles of Matter Have Space between Them
- Particles of Matter Are Continuously Moving
- Particles of Matter Attract Each Other
- States of matter
- The Solid State
- The Liquid State
- The Gaseous State
- Can matter change its state?
- Effect of Change of Temperature
- Effect of Change o Pressure
- Evaporation
- Factors Affecting Evaporation
- How Does Evaporation Cause Cooling?
- Physical nature of matter
Note: As per the revised CBSE curriculum, this chapter has been removed from the syllabus for the 2020-21 academic session.
States of Matter
- Matter can be classified as solid, liquid and gas on the basis of interparticle forces and the arrangement of particles.
- These three forms of matter are interconvertible by increasing or decreasing pressure and temperature. For example, ice can be converted from solid to a liquid by increasing the temperature.

Atomic view of the three states of matter


Evaporation
The phenomenon by which molecules in liquid state undergo a spontaneous transition to the gaseous phase at any temperature below its boiling point is called evaporation.
- For example, the gradual drying of damp clothes is caused by the evaporation of water to water vapour.
Factors affecting evaporation
- Temperature: The rate of evaporation increases with an increase in temperature.
- Surface area: The rate of evaporation increases with an increase in surface area.
- Humidity: The rate of evaporation decreases with an increase in humidity.
- Wind speed: The rate of evaporation increases with an increase in wind speed.
Cooling due to evaporation
During evaporation, the particles of a liquid absorb energy from the surroundings to overcome the inter-particle forces of attraction and undergo the phase change. The absorption of heat from the surrounding makes the surrounding cool.
For example, sweating cools down our body.
Physical Nature of Matter
- A physical property is that aspect of the matter that can be observed or measured without changing its nature or composition.
- It is independent of the amount of matter present.
- Physical properties include appearance, colour, odour, density, texture, melting point, boiling point, solubility, etc.
Characteristics of Particles of Matter
Matter
Matter is anything that has mass and occupies space.
- Everything that we can touch, see, hear, taste and also smell is matter.
- It is made up of really tiny particles which cannot be seen through the eye.
The particles of which the matter is comprised influence its state and properties (physical and chemical).
1. Particles of matter have spaces between them
- This characteristic is one of the concepts behind the solubility of a substance in other substances. For example, on dissolving sugar in water, there is no rise in water level because the particles of sugar get into the interparticle spaces between the water particles.
2. Particles of matter are always in motion
- Particles of the matter show continuous random movements due to the kinetic energy they possess.
- A rise in temperature increases the kinetic energy of the particles, making them move more vigorously.
3. Particles of matter attract each other
In every substance, there is an interparticle force of attraction acting between the particles. To break a substance we need to overcome this force. The strength of the force differs from one substance to another.
Diffusion
When the particles of matter intermix on their own with each other, the phenomenon is called diffusion. For example, spreading of ink in water.
- During diffusion, the particles occupy the interparticle spaces.
- The rate of diffusion increases with increase in the temperature, due to increase in kinetic energy of the particles.
Can Matter Change Its State?
Effect of change of temperature on state of matter
On increasing temperature, the kinetic energy of the particles of the matter increases and they begin to vibrate with a higher energy. Therefore, the interparticle force of attraction between the particles reduces and particles get detached from their position and begin to move freely.
- As a result, the state of matter begins to change.
- Solids undergo a phase change to form liquids.
- Similarly, liquids also undergo a phase change to form gases.
Melting point
The melting point of a solid is defined as the temperature at which solid melts to become liquid at the atmospheric pressure.
- At melting point, these two phases, i.e., solid and liquid are in equilibrium, i.e., at this point both solid state and liquid state exist simultaneously.
Boiling point
The boiling point of a liquid is defined as the temperature at which the vapour pressure of the liquid is equal to the atmospheric pressure.
Latent heat of fusion
It is the amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point.
Latent heat of vaporisation
It is the amount of heat energy that is required to change 1 kg of a liquid into gas at atmospheric pressure at its boiling point.
Sublimation
The transition of a substance directly from its solid phase to gaseous phase without changing into the liquid phase (or vice versa) is called sublimation.

Sublimation – Solid to Gas Phase Transformation
Effect of change in pressure on state of matter By applying pressure, the interparticle spaces between particles of matter decreases. Thus, by applying pressure and reducing temperature we can convert a solid to liquid and a liquid to gas.
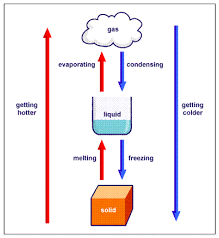
Flowchart for inter-conversion of the three states of matter




