In this post We will Study, The Solid State Notes & Important Topic for Student to excel in exam.
The Solid State NCERT Solution Class 12th Chemistry
NCERT/CBSE class 12th Chemistry notes provided by free-education.in (Wisdom Education Academy).
Here We are providing all subject wise pdf notes to student for their help to get good marks in exam.
In this post you will get Download CBSE 2020-21 Chemistry PDF notes given below by free-education.in to excel in the exam.
www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Engineering post, Career Guidelines , English Speaking Trick , How to crack interview and lots more.
Free pdf notes for Class 12th chemistry chapter – 1 ( The Solid State)
Chapter 1: The Solid State (Download pdf notes)
Here in this post we have made notes on most important topic of first chapter of chemistry (The Solid State)
However Let’s find out Here, what are important topic of Solid State for student to excel in exam.
Introduction: ( The Solid State Notes )
Everything in our surroundings is known as matter that can be categorized into three states.
In our day to day life solids plays a crucial role to pursue different purposes. Different types of solids with different properties are required for different purposes. The constituent particles and the type of bonds between the particles determine the nature of a specific solid. For eg. Bucket or a container used to carry water, utensils used for cooking food, computer, vehicles, electronic gadgets, notebooks, pencils, papers etc. are all solid substances used in our day to day life.

Liquids and gases on the other hand are another state of matter and are also known as fluids due to their ability to flow. They attain the ability to flow due to the free movement of molecules.
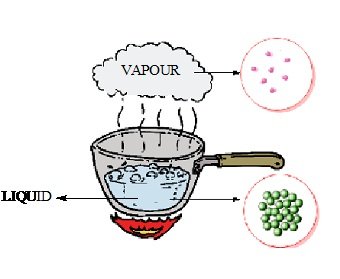
Fig. Particles of liquid (water) are loosely packed than solid and have space between them whereas particles of gas are loosely packed and have excess space between the particles
Let’s Understand What are Characteristics of solid state
- Definite shape due to strong intermolecular forces of attraction.
- They have distinct boundaries.
- Fixed volume.
- They cannot flow.
- Solid have negligible compressibility due to negligible distance between the neighbouring molecules.
- Solid possess a tendency to uphold their shape when exposed to external force.
- They break under force but it is difficult to change their shape so they are rigid.
- They have high density and do not diffuse at all.
The State of Solid: Solid is Classified into two categories:-
- Crystalline Solid
- Amorphous Solid
Crystalline Solid:-
- This range of solids consists of a broad range of small crystals having a definite characteristic geometrical shape.
- The constituent particles are arranged in a long range order (symmetry and regularity of arrangement of constituent particles that repeat at any distance from a given atom due to the interaction between the particles) with a regular and periodically repeating pattern over the entire crystal.
- Crystalline solids possess a sharp melting point.
- Crystalline solids are anisotropic in nature due to different arrangement of particles in different directions. This leads to different value of physical property along different directions in the same crystals.
- Metallic elements including iron, copper and silver are typical examples of crystalline solids.
- On the other hand non – metallic elements like sulphur, phosphorus and iodine and compounds like sodium chloride, zinc sulphide and naphthalene and quartz are typical examples of crystalline solids.

Amorphous Solid :-
- The term amorphous solid is derived form a Greek word amorphous meaning no form.
- The constituent particles are arranged in a short range order with a regular and periodically repeating pattern over short distances.
- These solids get softened at a certain temperature and hence can be moulded and drawn into various desired shapes. Solids may also acquire crystalline form at some temperature when heated.
- These solids have the ability to flow very slowly due to which they are also termed as pseudo solids or super cooled liquids.
- These solids are isotropic in nature due to the absence of long range order and irregular arrangement of the constituent particles in all direction. This leads to same value of physical property along all the direction.
- Irregular arrangement of particles in an amorphous solidGlass, rubber, amorphous silicon and plastics are typical examples of amorphous solids.
- Amorphous silicon is a photovoltaic material widely used for conversion of sunlight into electricity.
Molecular Solids
Solid composed of molecules as constituent particles. These solids can further be categorized into following types:
- Non polar Molecular Solids:
- They are composed of either atom.
- H2, Cl2 and I2.are some of the typical example.
- In non-polar Molecular solids the comprised atoms or molecules are held together by weak dispersion forces or London forces.
- They are soft and non-conductors of electricity.
- They have low melting points and usually exist in liquid or gaseous state at room temperature and pressure.

(ii) Polar Molecular Solids:
- The molecules like HCl, SO2, are formed by polar covalent bonds.
- In polar Molecular solids the comprised atoms or molecules are held together by stronger dipole-dipole interactions.
- They are soft and non-conductors of electricity.
- The melting points of these solids are higher than those of non-polar molecular solids and usually exist in liquid or gaseous state at room temperature and pressure.
- Molecules like SO2 and solid NH3 are some examples of such solids.

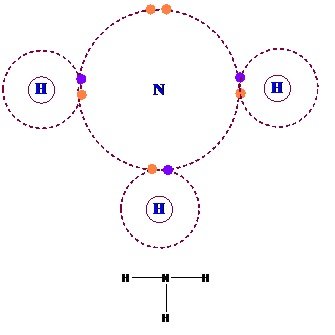
(iii) Hydrogen Bonded Molecular Solids:
- Hydrogen Bonded Molecular Solids contain polar covalent bonds between H and F, O or N atoms.
- Strong hydrogen bonding binds molecules of such solids like H2O (ice).
- They are non-conductors of electricity and are volatile liquids or soft solids under room temperature and pressure.

Ionic Solids
- The constituent particles of these solids are ions.
- The solid is composed of three dimensional arrangements of cations and anions that are bonded by strong coulombic (electrostatic) forces.
- These solids are hard and brittle in nature and have high melting and boiling points.
- They are electrical insulators in solid state due to the absence of movement of free electrons but are good conductors in molten state due to the movement of ions.

Metallic Solids
- In these solids positive ions is surrounded by mobile free electrons and are evenly spread all over the crystal.

- Each metal atom donates one or more electrons to the group of mobile electrons which increases the electrical and thermal conductivity of the metallic elements.
- Application of electric field makes these electrons flow through the linkage of positive ions.
- Whereas application of heat to one portion of a metal makes the thermal energy spread uniformly throughout by free electrons.
- Presence of free electrons in metals makes them lustrous, malleable and ductile. For example, Cr, Fe etc.
Covalent solids
- Crystalline solids of non-metals comprises of covalent bonds between adjacent atoms that are strong and directional in nature due to which atoms are held very strongly at their positions all over the crystal.

Fig. Covalent bonding between Carbon and hydrogen
- They are also called giant molecules.
- These solids are very hard and brittle.
- They have extremely high melting points and may decompose before melting.
- They are insulators and do not conduct electricity.
- Diamond and silicon carbide are typical examples of such solids but Graphite is an exception as it is soft and a good conductor of electricity.

What makes graphite soft and a good conductor of electricity?
- Graphite is a covalent solid that act as a good conductor of electricity and is soft.
- The carbon atoms of graphite are prescribed in different layers and are covalently bonded to three of its surrounding atoms in the same layer.
- The fourth valence electron of each atom is present between different layers and is free to move which makes graphite a good conductor of electricity.
- The characteristic of sliding between different layers makes graphite a soft solid and a good solid lubricant.
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]






