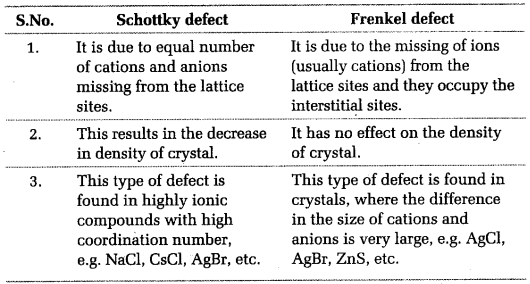
Imperfections in Solids
- Constituent particles in crystalline solids are arranged in a short range as well as long range
order yet crystals are not perfect. - They possess small crystals have defects that are irregularities in the arrangement of constituent
particles in them when crystallization process occurs at fast or moderate rate. - These defects are of two types- point defects and line defects.
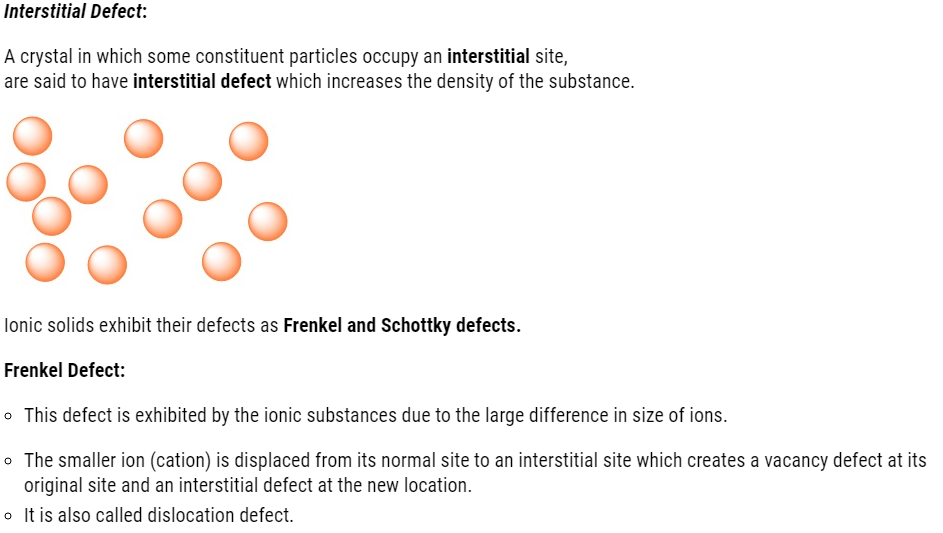
- The irregularities or deviations from ideal arrangement around a point or an atom in a
crystalline substance. - The irregularities or deviations from ideal arrangement in entire rows of lattice points are known as line defects.
- These irregularities are called crystal defects.
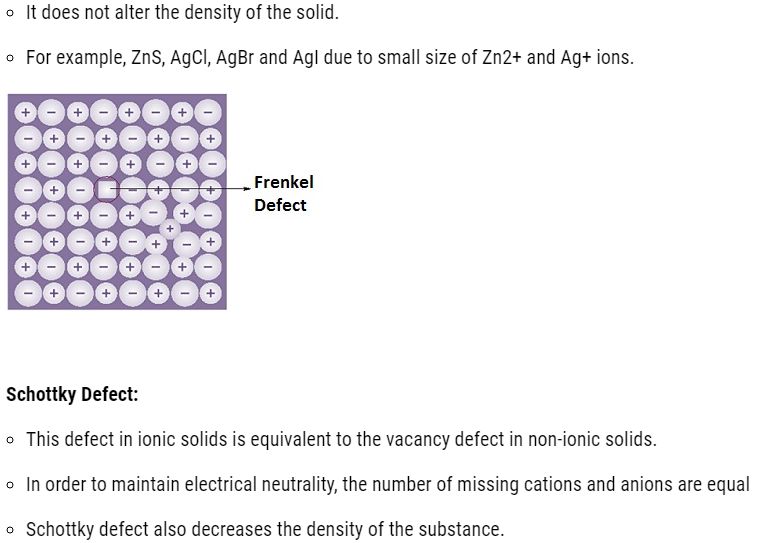
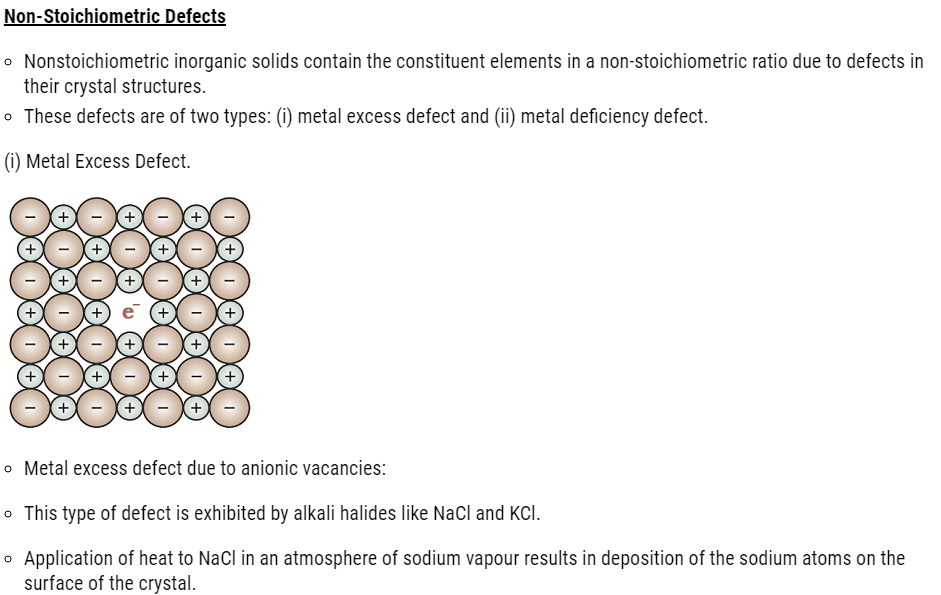
- Point defects can be classified into three types: (i) stoichiometric defects
(ii) impurity defects and (iii) non-stoichiometric defects
Defects in Solid






Electrical Properties of soilds
Solids exhibit electrical conductivities that extend from 27 orders of magnitude ranging from 10–20 to 107 ohm–1 m–1.
Conductors:
- Solids with conductivities ranging between 104 to 107 ohm–1m–1 are conductors.
- Metals have conductivities in the order of 107 ohm–1m–1 are good conductors.
- For example, Iron, Copper, Aluminum.

A man touching the electric pole with a metal rod will get an electric shock because metal rod is a conductor whereas a man touching the same with a wooden plank will be safe because wood is an insulator.
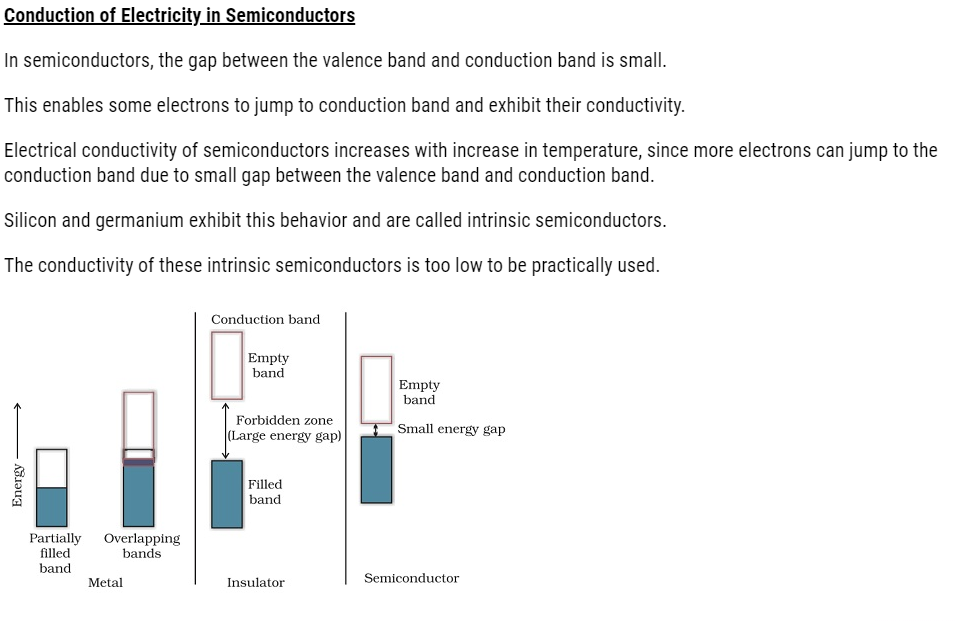
- Semiconductors:
- Solids with conductivities in the intermediate range from 10–6 to 104 ohm–1 m–1.
- For example, Gallium, Germanium, Silicon

- Metals conduct electricity both in solid state as well as molten state.
- The conductivity of metals depends upon the number of valence electrons available per atom.
- The atomic orbitals of metal atoms form molecular orbitals that are close in energy to each other as to form a band.
- Partial filling or overlapping with a higher energy unoccupied conduction band enables the electrons to flow easily under an applied electric field.
- This results in conductivity of metals.
If the gap between valence band and the conduction band is large, electrons cannot jump to it and such a substance has very low conductivity that makes it behave like an insulator

Number of cation vacancies in lattice of NaCl = Number of divalent Sr2+ ions added.
Concentration of cation vacancy on doping with 10-3 mol% of SrCl2. = 10-3 mol% = 10-3 / 100 = 10-5 mol.
Number of Sr2+ ion in 10-5 mol = 10-5 x 6.023 x 1023 = 6.023 x 1018
Hence number of cationic vacancies = 6.023 x 1018
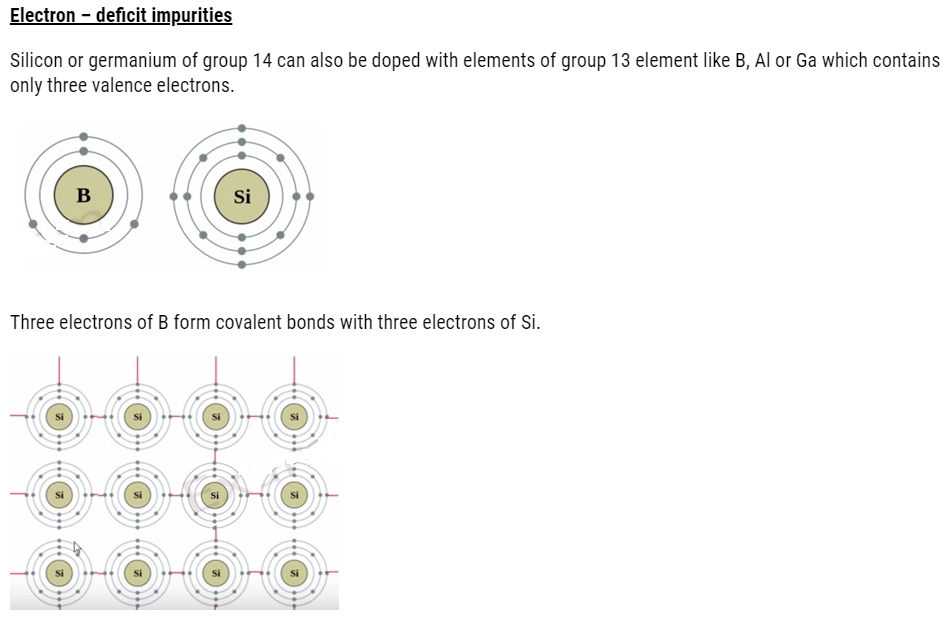
Electron – rich impurities
In a periodic table Silicon and germanium belongs to group 14 with four valence electrons each. In their crystals each atom forms four covalent bonds with surrounding atom.
When doped with a group 15 element like P or As, which contains five valence electrons,

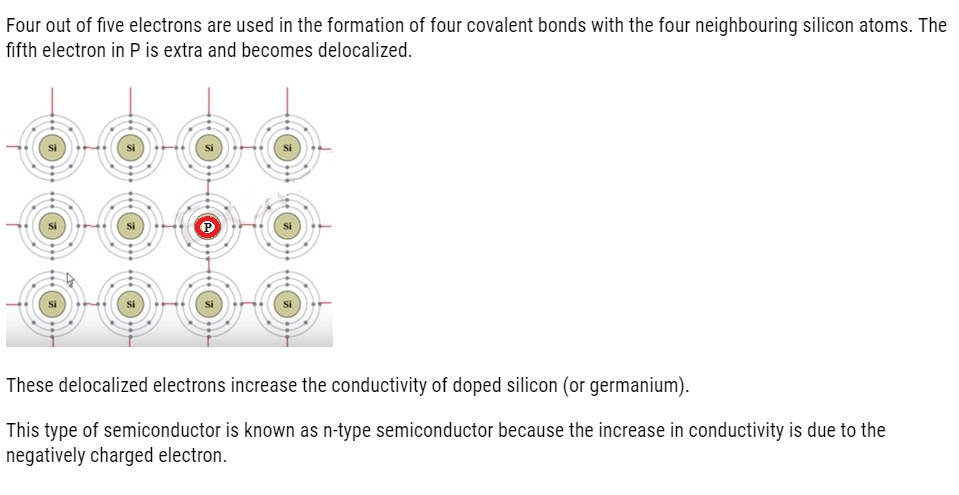
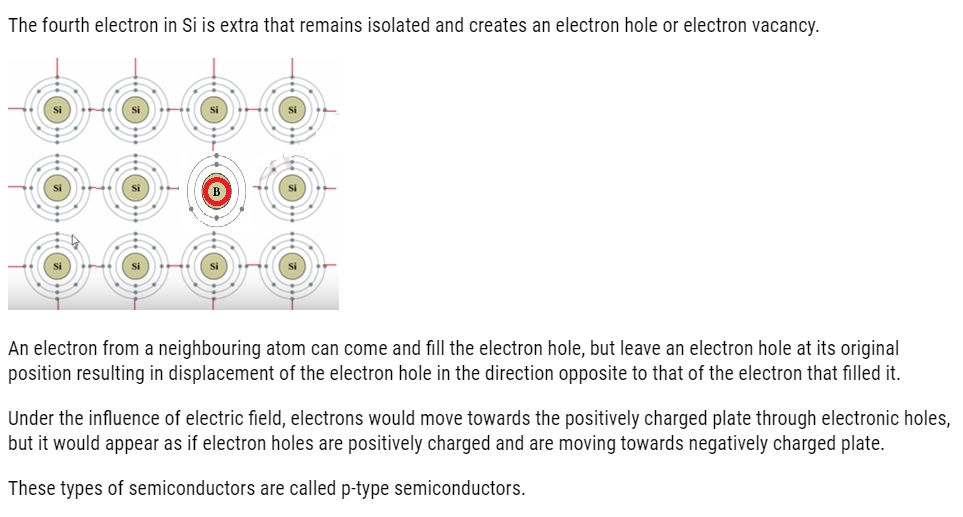
Applications of n-type and p-type semiconductors
- n-type and p-type semiconductors finds a great use in manufacturing electronic components.
- Diode is a combination of n-type and p-type semiconductors extensively used as a rectifier.
- Transistors are manufactured by keeping a layer of one type of semiconductor between two layers of another type of semiconductor.
- npn and pnp type of transistors are used to detect or amplify radio or audio signals.
- The solar cell is an efficient photo-diode used for conversion of light energy into electrical energy.
- Gallium arsenide (GaAs) semiconductors have very fast response and have transformed the design of semiconductor devices.
- Transition metal oxides show marked differences in electrical properties.
- TiO, CrO2 and ReO3 behave like metals.
- Rhenium oxide, ReO3 resembles metallic copper in terms of its conductivity and appearance.
- Certain other oxides like VO, VO2, VO3 and TiO3 exhibit metallic or insulating properties depending on temperature.
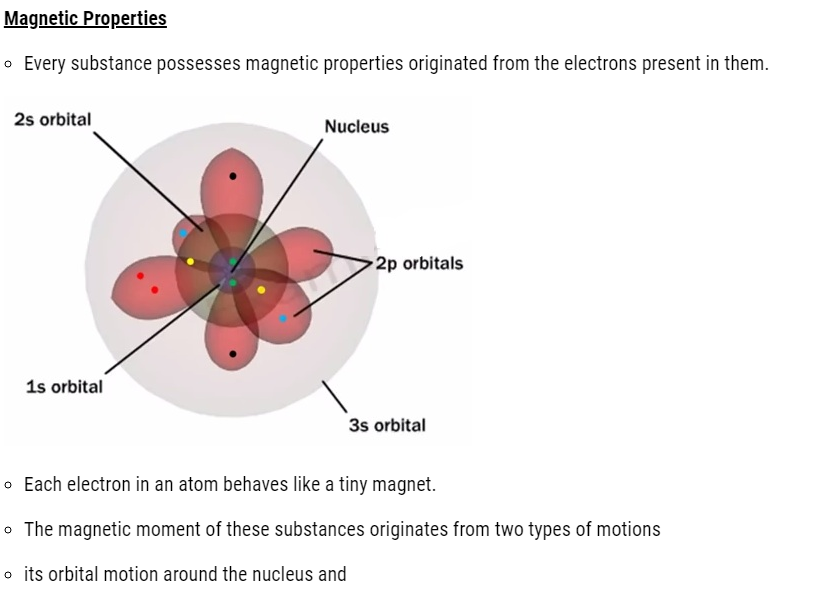
- its spin around its own axis.
- Electron being a charged particle undergoes these motions and can be considered as a small loop of current possessing a magnetic moment.
- Therefore, each electron has a permanent spin and an orbital magnetic moment associated with it.
- Magnitude of this magnetic moment is very small and is measured in the unit called Bohr magneton, μ B and is equal to 9.27 × 10–24A m2.
On the basis of their magnetic properties, substances can be classified into five categories:
- Paramagnetic
- Diamagnetic
- Ferromagnetic
- Antiferromagnetic
- Ferrimagnetic.


Ferromagnetism
- Ferromagnetic substances get strongly attracted towards magnetic field.
- They can be permanently magnetized.
- In solid state, the metal ions of ferromagnetic substances are grouped together into small regions and are known as domains that act as a tiny magnet.
- In an unmagnetised ferromagnetic substance the domains are randomly oriented that cancels out their magnetic moments.
- When placed in a magnetic field all the domains of the substance get oriented in the direction of the magnetic field producing a strong magnetic effect which persists even on removal of the magnetic field and the ferromagnetic substance becomes a permanent magnet.
- For example, iron, cobalt, nickel, gadolinium and CrO2 are ferromagnetic substances.


For Full Chapter Notes :
Download the Previous Year Question Papers
Let’s Know the Interesting facts about Indian President
Most Interesting Cricketing Facts & Records.
Do You want to know about Indian Space agency and ISRO, Lets Find Out..
Also Learn Daily Use English Sentences to Impress your friends
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]






