www.free-education.in is a platform where you can get pdf notes from 6th to 12th class notes, General Knowledge post, Engineering post, Career Guidelines, English Speaking Trick, How to crack an interview, and lots more. ( Class 11 Chemistry Chapter 11 The p Block Elements Notes and NCERT Solution )
NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
Chapter 11 The p-Block Elements:
| Section Name | Topic Name |
| 11 | The p-Block Elements |
| 11.1 | Group 13 Elements: The Boron Family |
| 11.2 | Important Trends and Anomalous Properties of Boron |
| 11.3 | Some Important Compounds of Boron |
| 11.4 | Uses of Boron and Aluminium and their Compounds |
| 11.5 | Group 14 Elements: The Carbon Family |
| 11.6 | Important Trends and Anomalous Behaviour of Carbon |
| 11.7 | Allotropes of Carbon |
| 11.8 | Some Important Compounds of Carbon and Silicon |

Introduction of p- block
- P block is present at extreme right of periodic table.
- It has general electronic configuration ns2np1-6.
- It includes solids, liquids and gases.
- The elements of this group are metal, non- metal and metalloids.
- If we move along period in periodic table, non-metallic character increases and if we move down the group, the non-metallic character decreases.
- In this group: As we move down, the lower oxidation state becomes more stable due to” Inert pair effect.”
Inert pair effect : It is “reluctance in the participation of s electrons in bond formation due to poor shielding effect by d and f orbital .As a result, s electrons are tightly bounded .
1st member is different from its congeners due to:
- Small size
- Highest ionization energy.
- High electro-negativity.
- No vacant d orbital.
- They show maximum co-valence of 4 because of no vacant d orbital.
- First member also has tendency to form multiple bonds because in them p can takes place (because of its small size) .

Physical properties of group 13:
- Atomic size and Ionic radii:
- If we compare group 11 with group 13, then group 13 is smaller due to increased nuclear charge.
Example: Out of Be and B ,Boron is smaller .
- Down the group, size increases because nuclear charge decreases (due to addition of new shell).
- Expected order : B <Al<Ga<In<Tl
- But actual the order is :BGa<In<Tl
- The reason behind it: Is due to filling of d-orbital in Gallium, the effective nuclear charge on valence electron increases (because of poor shielding effect by d and f orbital).
- Therefore, nucleus pull electron more effectively and size decreases.
- That is the reason, Gallium is smaller than Aluminum (because Gallium has d-electrons and Aluminum doesn’t have) .
- Ionization energies
- If we compare ionization energy of group 13 and group 2, we can say that the ionization of group 13 is more, because of their small size and increased nuclear charge.
- But actually group 2 has high ionization energy, than group 13 due to completely filled s orbital in group 2 elements.
- In case of Be and B, the electronic configuration of Be is 1s22s2 and in boron it is 1s22s22p1. So, due to this reason the ionization energy of Boron is less than Beryllium.
Down the group ionization energy decreases, as the size increase and nuclear charge decrease.
So , the expected order is : B>Al>Ga>In>Tl
But actually it is : B>AlIn>Tl due to poor shielding effect by d electrons in gallium.
- Electronegativity: If we compare with group 2, they are more electronegative due to increased nuclear charge.
Along group: It decreases, but the expected order is this B>AlIn>Tl.
This is due to poor shielding effect.
- Electropositive character: As compared to group 2, they are less electropositive due to increased nuclear charge.
Down the group: The size increases. Therefore, metallic character increases.
B Al Ga In Tl
Non metal amphoteric less metallic metallic metallic
- Oxidation state: It depends on electronic configuration .As their electronic configuration is ns2 np.So, oxidation states shown by them are +3,+1.
- Boron: Shows exceptional behavior because of its small size and high ionization energy .It actually faces difficulty in loosing electrons .Therefore, it forms covalent bond.
B Al Ga In Tl
(+3) (+3) (+3,+1) (+3,+1) (+1)
This is basically due to inert pair effect.
Chemical properties of group 13
- Out of all elements of this group, Boron is non-reactive. This is because of its small size as it has high ionization energy .So, reactivity increases down the group.
B < Al < Ga < In < Tl
Boron aluminum Gallium indium thallium
- In this Boron is least reactive because of its small size and high ionization energy and Thallium is most reactive because of its low ionization energy.
- Aluminum: When react initially, it keeps reacting .But after some time, a layer of its oxide is formed over its surface. This layer prevent it from further reacting .So, it becomes passive after some time.
- Most of the compounds of this group are electron deficient that is their octet is not complete. So, they behave as Lewis acids.
Example: BX3 (only 6 electrons in valence shell of Boron).
Therefore, it forms coordinate bond.
BX3 + NH3–>[NH3–>BF3]coordinate bond
Boron halide ammonia
- That means, they have tendency to take electrons. Or, we can say they are Lewis acids. But down the group, acidic character decreases (that is tendency to attract incoming electron decreases).
BX3> AlX3 > GaX3 >InX3 >TlX3
boron halide aluminum halide gallium halide indium halide thallium halide
- Lewis acid strength decreases because size increases .As a result, attraction towards incoming electron decreases.
- Reactivity towards oxygen : Forms oxides
- Boron does not react with oxygen at ordinary temperature due to small size and high ionization energy.
- If we react Al with oxygen, it reacts at normal temperature. With time it forms a protective layer of oxide on its surface. This layer makes it non reactive.
Al +3O2 –>2Al2O3
aluminum oxygen aluminum oxide
- They react with nitrogen gas also, to form compound with formula EN.
Example: 6Al +3N2–>6AlN
aluminum nitrogen aluminum nitride
- If we see acidic strength of oxides, then it decreases down the group.
BeO Al2O3 GaO InO TlO
beryllium oxide aluminum oxide gallium oxide indium oxide thallium oxide
(acidic) (amphoteric) (all are basic)
- Reaction with water: Hydroxides are formed of type E(OH)
- Boron does not react with water.
- Aluminum reacts with cold water that is :
Al + H2O –> Al(OH)3 + H2
Aluminium water aluminium hydroxide hydrogen gas
- Gallium and indium neither react with cold water nor with hot water.
- Thallium reacts with water but form protective layer which make it passive.
- 3. Reactivity towards acids and bases:
- Boron doesn’t react with acids and bases at normal temperature, but reacts with strong acids.
- Aluminum reacts with acid and base because it is amphoteric in nature.
Al + HCl–>AlCl3 + H2
aluminum hydrogen chloride aluminum chloride hydrogen gas
Al + NaOH –>[NaAl(OH)4]
Aluminum sodium hydroxide sodium tetrahydroxoaluminate
- Al when react with nitric acid, initially it reacts but after same time it became passive.
- Reaction with Halogens: Form halides of type EX3
2B +3X2–>2BX3
boron halogen boron halide
B +F2 –>BF3
boron halogen boron halide
Al +Cl2àAlCl3
aluminum chlorine aluminum chloride
All halides are Lewis bases.
Important trends
- Formation of Hydrides : EH3
- Thermal stability of hydrides decrease down the group .Out of all, only BH3 is stable.
BnHn+4 , BnHn+6= Boranes
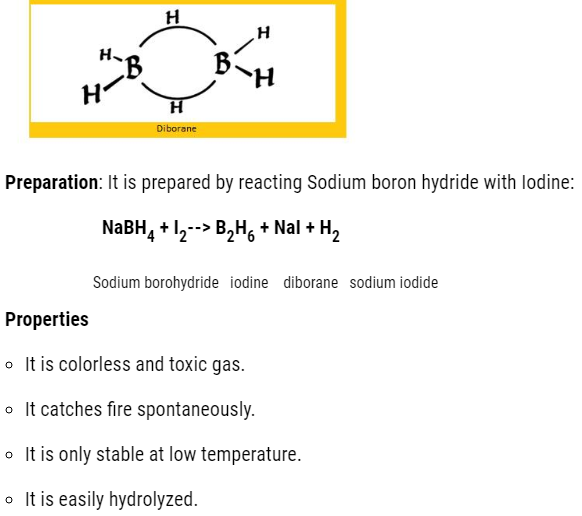
Simplest Borane :B2H6(diborane )
- This diborane has banana bond and it is weak Lewis acid.
- Formation of Halides: They form their respective halides. Out of all halides aluminum chloride AlCl3exist as dimer that is Al2Cl6 (all halides behave as Lewis acids)
- They will behave as Lewis acids only, if they attract incoming electrons.
- Trends of strength of Lewis acids :
- BF3>AlCl3>BBr3>BI3(expected order)
- BF3<AlCl3>BBr3>BI3(actual)
Explained on the basis of back bonding:
- In case of BF3, in Boron there is one vacant 2p orbital ( i.e 2s2,2p1)in ground state.
- In excited state, it will be 2s1, 2p2 and energy of 2p orbital of B and F are almost similar.
- As a result, one of the 2p filled orbital overlaps sidewise with the vacant 2p orbital of boron atom resulting in transfer of electrons from Fluorine to vacant 2p orbital.
- This is called back bonding.
- Now it doesn’t have fewer electrons therefore, Boron no longer act as a Lewis acid.
- As size increases, the tendency of back bonding decrease and acidic strength increase.
Uses of Boron
- Boron is used as semi conductor for making electrical appliances.
- It is used in steel industry for hardening.
- Its compounds like borax and boric acid are used in glass industry.
- Borax is used for soldering metals.
- Borax fibers are used in making bullets.
Uses of Aluminum
- It is soft and light metal, non toxic and is used for wrapping food items.
- It I used in making electric power cables.
- It is used as packaging of food items.
- It is used I making cans for cold drink etc.
Alloys of Aluminum
- Bronze : Aluminum and Copper are its constituents .It is used for making coins ,jewellery etc
- Magnalium: its constituents are Al and Mg.
It is used for making pressure cookers, balance beams etc.
- Duralumin: its constituents are: Al, Cu, Mg and Mn.
It is used for making bodies of air craft’s, helicopters, ships etc.
- ALNICO: Its constituents are AL, Ni and Cobalt.
It is used in making powerful magnets.
Borax
- Borax: Na2B4O7.10H2O

Preparation
- From Colemanite ore: It is prepared from Colemanite ore (calcium ore).
- In this ore is made to react with sodium carbonate.
- Then it is heated as shown in reaction :
Ca2B6O11 + Na2CO3—> CaCO3 +Na2B4O7 + NaBO2
Colemanite ore sodium carbonate calcium carbonate Borax sodium metaborate
- The solution has White precipitate.
- When these precipitates are filtered, the solution becomes concentrated.
- Finally, we get crystals of borax on cooling.
- From Boric acid : In this also boric acid is made to react with Sodium carbonate to form Borax ,carbon dioxide and water as shown below:
H3BO3 + Na2CO3—>Na2B4O7 + CO2 + H2O
Boric acid sodium carbonate borax carbon dioxide water
PROPERTIES OF BORAX
- It is white crystalline solid.
- On heating, it loses water of crystallization and form Na2B4O
- On further heating, it gives white transparent liquid which further on cooling gives white transparent bead.
- This bead is made to react with different types of salts.
- When reacted ,it gives different colour with different metal ions like :
With Ni2+–>brown
With Co2+–>blue
With Cr3+àgreen
With Mn2+–>pink
With Cu2+–>blue
Uses of Borax
- It is used in laboratory as Borax bead test.
- It is used in making enamels for pottery.
- It is used in candle making.
- It is added in soaps due to its antiseptic properties.
- It is used in optical glass.
- Ortho boric acid
Chemical Formula :- H3BO3 or B(OH)3
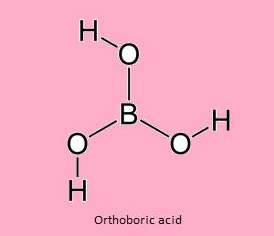
Preparation: It is prepared from Borax.
Na2B4O7.10H2O + HCl +H2O–>H3BO3 + NaCl
Borax hydrochloric acid boric acid sodium chloride
Properties
- It is white crystalline solid with soapy touch.
- It is sparingly soluble in cold water but soluble in hot water.
- At 373k, it forms metaboric acid.

- Boron hydrides
The molecular formula is BnH2n+4
The common hydride is :B2H6 that is diborane.
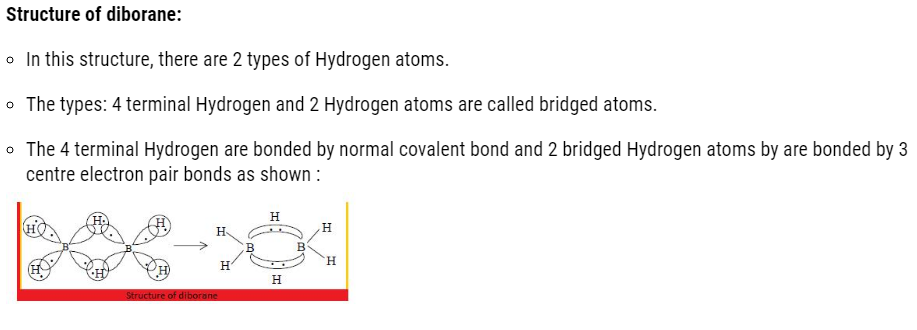
- Both the Boron will have in total 2 empty d orbital.
- Each 2p orbital overlaps with 1s orbital of H atoms.
- The 2 hybrid orbital left on each Boron atom contain an unpaired electron and other is empty.
- The orbital containing one electron of one boron atom and the other empty orbital of second boron atom form a bond with hydrogen atom simultaneously to give B-H-B bond.
- Each Boron form 2 covalent bond.
- Out of 3 unpaired electrons, the left electron of both the boron is 2 and of 2 Hydrogen atom is also 2.
- Therefore, it forms 3 bonds instead of 2 bonds.

Physical Properties of group 14
- Atomic size: If we compare its size with group 13, then size of group 13 elements is bigger than group 14. As group 14 elements are smaller due to increased nuclear charge.
Along group: As we move down size increases, as each time a new shell is being added.
- Ionization energy: It is amount of energy required to remove electron from last shell of an atom ,when it is in gaseous state .We know ionization energy is inversely proportional to size therefore , ionization of group 14 is higher than group 13, due to its small size .
Along group: Ionization energy decreases because size increases. If we look at the trend of ionization energy we see certain abnormality:
C > Si > Ge >Sn <Pb
Carbon silicon germanium Tin lead
As we move from tin to lead ,the ionization energy increase due to poor shielding effect of 4 f orbital in Lead .
- Melting point and Boiling point
The boiling point of group 14 is higher than group 13 .As they form covalent bonds because of their small size.
As we move down the group melting and boiling point decreases due to increase in size, bonds formed are not so strong.
C > Si > Ge >Sn >Pb
Carbon silicon germanium Tin lead
4 .Metallic character
- The tendency to lose electrons depends upon ionization energy or, we can say that less is the ionization energy, more is the metallic character.
- If we compare for group 13 and group 14, we see that group 13 is more metallic due to big size and low ionization energy.
- Down the group metallic character increases as size increase and ionization energy decreases. Therefore, the order is :
- C Si Ge Sn Pb
- Carbon silicon germanium Tin lead
(non metals ) (metalloid ) (metals )
- Oxidation states
This group can show oxidation states +4 and +2.
- Carbon: Due to high ionization energy, sharing is preferred in case of it. So, oxidation state shown is 4.
- Silicon,Ge, Tin : They commonly show +4.
- Lead: Show +2 due to inert pair effect.
- All these elements have special property that is,if they are present in +2 oxidation state they act as reducing agents .
Example: Tin (Sn+2)

Chemical properties of group 14
They are non reactive but reactivity goes on increasing down the group due to decrease in ionization energy.
- Reactivity towards oxygen : They form two types of oxides
- Monoxides (MO)
- Dioxides (MO2)
That is :
- Monoxides : CO,SiO,GeO,SnO,PbO
- Dioxides: CO2,SiO2,GeO2,SnO2,PbO
Out of them:
- Co: Neutral
- SiO: is not so stable
- GeO: Weakly acidic
- SnO and PbO : Amphoteric
- CO2,SiO2 : Acidic
- GeO2: Amphoteric
- SnO2 and PbO2: weakly basic
Out of them, CO Is strongest reducing agent because it has ability to accept oxygen and form stable oxide that is carbon dioxide .The solid form of carbon dioxide is called dry ice and the commercial name of dry ice is drikold. Out of them PbO2 is strongest oxidizing agent .
- Reaction with water: They form hydroxides.
- In this group, Carbon does not react with water.
- Tin reacts with steam forming SnO2+H
- Ge,Sn Pb –do not react with water due to formation of protective layer of oxide on it .
- Reaction with halogens: Halides are formed (EX4).
The halides formed are :
CCl4SiCl4 GeCl4 SnCl4 PbCl4
SnCl2 PbCl2
All are tetrahedral in nature.
Structure of CCl4:

- Reaction with hydrogen : hydrides are formed (EH4)
They form respective hydrides:
- CH4 SiH4 GeH4 Sn and Pb do not form as they are less reactive towards hydrogen.
- Carbon has maximum tendency to form hydrides in its own family .these hydrides have covalent bonding in them and a tetrahedral geometry.
Allotropes of carbon
Allotropes: Are the different forms of elements having same physical properties but different chemical properties.
Allotropes of carbon
- Crystalline form :Diamond ,Graphite and Fullerene
- Amorphous forms of carbon : Coke ,Charcoal ,lamp black
- Diamond
In this carbon is sp3 hybridized .Each carbon attached to four carbon atom giving rise to compact three dimensional structures given below:
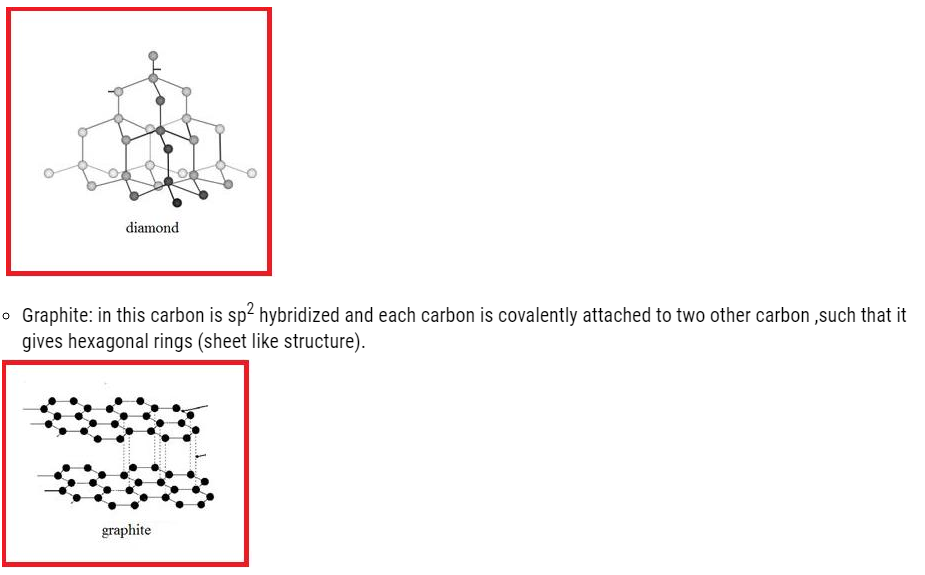
- The layers are held by weak Vander wall forces, such that they can slide over one another.
Properties and Uses of diamond and graphite
- Diamond
- It is hardest substance. Therefore, used as cutting tool.
- It is bad conductor of electricity because it has no free electrons.
- Graphite: It has soft structure because of Vander wall forces in it.
- Therefore, used as Lubricant.
- It is used to make pencil leads, as it marks the paper black.
- Moreover, it is good conductor of electricity as it has free electrons.
Uses of Diamond
- It is used as cutting tool.
- It is used in making jewellery.
- It is used in manufacturing of tungsten filament.
Uses of Graphite
- It is making electrodes.
- It is used as lubricant
- It is mixed with clay or wax to make lead pencils
- It is used to making moderator of nuclear reactor.
Both Diamond and Graphite are crystalline forms of Carbon.
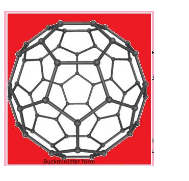
- Fullerenes: It is having many Carbon atoms ranging from C32 to CIt was studied in 1985 and after 10 years it came in notice or structure. The carbon atoms are in a shape of football .
Buckminster form :
- Amorphous forms of Carbon : In this the molecules are arranged in haphazard manner .
- Coke: Used as fuel.
- Charcoal : Is porous
Types of Charcoal:
- Wood charcoal
- Animal charcoal
- Sugar charcoal
(They are obtained by destructive distillation of wood, sugar etc).
- Charcoal is good absorbent, if dipped in colored solution it will adsorb all colours, leaving behind colorless solution.
Uses of Carbon
- It is used in the form of fuel.
- It is used in manufacturing of coal gas, water gas etc.
- It is also used as a good reducing agent in metallurgy.
- It is activated charcoal and is used as catalyst.
Uses of Silicon
- It is used to form n-type or p-type semiconductor.
- It is important component of glass and cement.
- Pure Si is used to make computer chips.
Uses of germanium
- It is used in transistors.
- It is making for lenses and prism.
- It is used as scientific apparatus.
Uses of lead
- It is used for making lead sheets and pipes.
- It is used for telephone wires.
- It is used in storage batteries and bullets.
Uses of Tin
- It is used for electroplating.
- It is used in making alloys: Pb, Cu and Sn.
- It is a type metal: Pb ,Sn ,Sb.
Carbon monoxide (CO)
Preparation
- It is prepared from incomplete combustion of carbon as given in reaction below :
C + ½ O2 –>CO
Carbon oxygen carbon monoxide
- Heating Methanoic acid in presence of sulphuric acid at temperature 473-1273 k.
HCOOH + H2SO4— >CO + H2O
Methanoic acid sulphuric acid carbon monoxide and water
- Commercial preparation: Heating Coke with water at high temperature.
C + H2O –> CO + H2
Carbon water syn gas
If we mix carbon in air :
C + O2+ 4N2 –> CO + N2
carbon oxygen nitrogen carbon monoxide and nitrogen gas
Properties
- It is colourless and odourless.
- It is insoluble in water.
- It is highly toxic in nature as it combines with Haemoglobin,to form defective complex .As a result, the oxygen carrying capacity of RBC gets reduced.
- It burns in air to form carbon dioxide.
Uses
- It acts as reducing agent in extraction of metals.
- It reacts with certain metals to form Metal carbonyls.
- It forms synthesis gas that has industrial advantage.
- It forms producer gas that has industrial advantage.
Carbon dioxide (CO2)
Preparation
- From complete combustion of Carbon and Carbon containing fuels in excess of air:
C + O2 — >CO2
carbon oxygen carbondioxide
CH4+ O2 –.>CO2 + H2O
Methane carbon dioxide and water
Properties
- It is colourless and odourless.
- It is soluble in water.
- It is non supporter of combustion.
- It combines with water to for Carbonic acid (weak dibasic acid).
Uses
- It forms carbonic acid which undergo dissociation as :
This H2CO3 and HCO3– helps in maintaining pH of blood between 7.26 to 7.42.
- Carbon dioxide is used by plants for photosynthesis: If the percentage of this gas increases in atmosphere, it has adverse affect like green house effect, which further results in ecological imbalance .
- Dry ice (solid form of carbon dioxide) is used as refrigerant for ice creams and frozen food.
- Gaseous carbon dioxide is used in carbonated soft drinks.
- Carbon dioxide is used in fire extinguisher as it is non supporter of combustion.
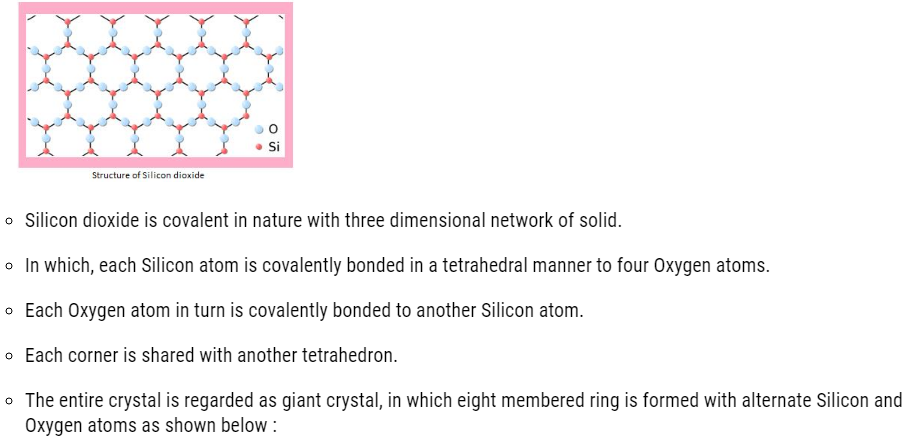
Silicon dioxide (SiO2)
About 95% of earth crust is made up of silica and silicates .Silicon dioxide is commonly called as silica and it occurs in different forms :
Crystalline form of silica
- Quartz
- Cristobalite
- Tridymite
These forms are inter-convertible at suitable temperature.
Structure of Silicon dioxide
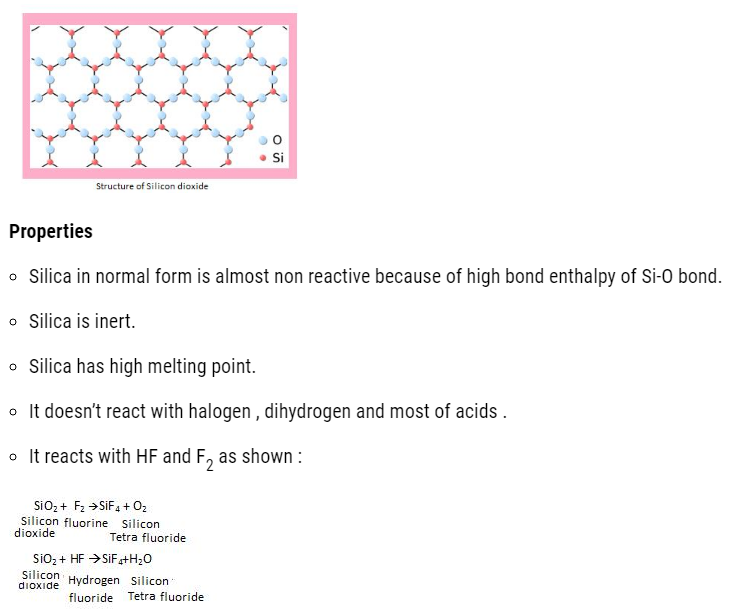
- This silicon tetra fluoride can easily dissolves in HF to form H2SiF6 (hydro-Fluoro-silicic acid ).
- Due to formation of this compound, the SiO2 present in glass gets dissolved. That is the reason the hydrogen fluoride can’t be stored in glass bottles, instead it is stored in containers made of Cu, Ni+ traces of Fe alloy .
- Quartz is used as piezoelectric material.
Uses
- Silicon dioxide is used as catalyst in petroleum industry.
- When (NH4)2CoCl4 is added to silica gel , it act as a humidity detector .Since ,it is blue when dry but turns pink when hydrated .
Silicones
- They are synthetic organo-silicon compounds containing repeated R2SiO units held by Si-O-Si linkages.
- These compounds have the general formula (R2SiO)n where R is methyl or aryl group .
Preparation
- The methyl chloride reacts with Silicon in presence of Copper at temperature 573k.
- As a result, we get different types of methyl substituted cholrosilane of formulas :MeSiCl3, MeSiCl2, Me3SiCl and also Me4
CH3Cl + Si –> (CH3)2SiCl2
methyl chloride dichloromethylsilane
(CH3)2SiCl2 + H2O –> (CH3)2Si(OH)2
Dichloromethylsilane
- If we carry out hydrolysis of dichlorodi-methylsilane followed by polymerization we get , straight chain polymers
Properties
- Silicones with :
- Short chains – oily liquids
- Medium chain- viscous oils, jellies and greases
- Long chains – rubbery elastomers and resins .
- They are chemically inert, resistant to oxidation and thermal decomposition.
- Silicones are surrounded with non polar alkyl group that are water repelling in nature.
- They are heat resistant and possess high dielectric constant.
Uses
- They are used in making water proof papers, wool ,textile, wood etc by coating them with thin film of silicones.
- They are used as electric insulators.
- They are used as lubricants at high as well as at low temperature , as there is very little change in their viscosity with temperature.
- They are used in surgical implants.

Zeolites
- They are widely used as catalyst in petrochemical industries for cracking of hydrocarbons.
- In them basically the Silicon atoms in three dimensional structures is replaced by Aluminum ions.
- As a result, the overall structure carries the negative charge .
- To balance this negative charge some cations like sodium ion etc are added in the structure.
For example:
- ZSM-5 a type of zeolite converts alcohols directly to gasoline.
- Hydrated zeolite is used as permutit in ion exchange method for softening of hard water.
Related link you must like:-
Study material for Competition Exam
Mohd. Sharif Qualification: B.Tech (Mechanical Engineering) [Founder of Wisdom Academy] [Aim Foundation & Free-Education.In] [Engineer By Profession | Teacher By Choice] [Blogger, YouTube Creator]






