CBSE Class 6 Science Notes Chapter 4 Sorting Materials into Groups
There is a vast variety of objects everywhere.
All objects around us are made up of one or more materials.
A thing can be made of different materials.
Many things can be made from the same material.
Classification: The process of sorting and grouping things according to some basis is’called classification.
Basis of grouping: We can group materials on the basis of the similarities and differences in their properties.
Each material has its own properties, that may differ from others. However, some properties may be common to one or more materials.
Uses of a material: Uses of a material depend on its properties and the purpose for which it has to be used.
Metals have lustre while non-metals generally have no lustre.
Smooth and rough surface: Some materials feel smooth when touched e.g., mirror and things made of metals; whereas, some other materials feel rough when touched; for example, a piece of stone.
Hardness and softness: Materials which can be pressed easily are called soft while some other materials which cannot be pressed are called hard; for example, cotton is soft while wood is hard.
Solubility
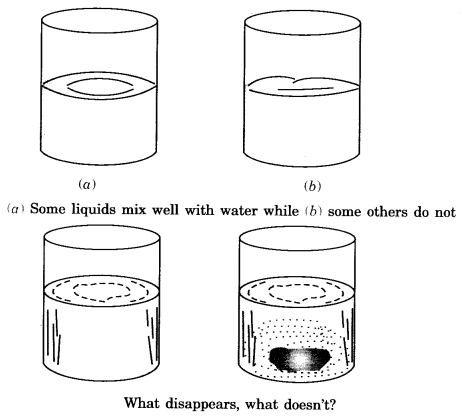
- Soluble substances: Substances that get dissolved in water are called soluble substances.
- Insoluble substances: Substances which do not dissolve in water are called insoluble substances.
- Miscible: Those liquids which mix well with water are said to be miscible.
- Immiscible: Substances which do not mix well with water are called immiscible.
- Solubility of gases: Solubility of gases in water is very less.
- Oxygen gas which is dissolved in water, is very important for the survival of aquatic plants and animals.
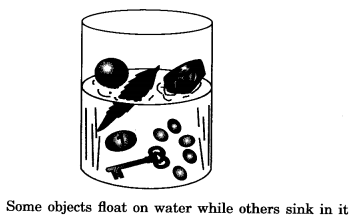
Buoyancy: Some materials float in water while others sink:
- Materials like sand, sugar and salt sink in water.
- Materials like wax, oil and wood float on water.
Transparency
- Opaque: Materials through which we are not able to see are called opaque. For example, wood, iron, gold.
- Translucent: Materials through which things are only partially visible are called translucent. For example, butter paper, old glass door.
- Transparent: Materials through which things can be seen are called transparent. For example, glass, water, air, test tube.
Things are grouped together for convenience and to study their properties.
Hard: Materials which cannot be pressed easily are called hard e.g., stone.
Insoluble: Materials which do not dissolve in water are called insoluble.
Lustre: Some materials have a special shine on them which is called lustre.
Material: The matter of which an object is made, is called material. For example, glass, steel, wood, etc.
Metals: Materials which have certain properties like, lustre, malleability, ductility and are sonorous, good conductors of heat and electricity, are called metals.
Opaque: Materials which do not allow light to pass through them are called opaque.
Rough: Some materials have uneven surface and feel rough on touching.
Soluble: Materials which dissolve in water are called soluble.
Translucent: Materials which partially allow light to pass through them are called translucent.
Transparent: Materials which allow light to pass through them are called transparent.
Grouping On The Basic Of Common Properties:
Objects are made of different materials. One material can be used to make different objects. This is possible because different types of materials have different properties. We have to choose materials with the right properties based on what we want to use it for. For example, a chalk made of wood or plastic would be of no use because it cannot be used to write on the blackboard.
Materials have different properties like roughness, lustre, transparency, solubility, flotation, attraction towards a magnet, conduction of heat, and conduction of electricity. Let us study these properties one by one.
Roughness:
Materials can be rough or smooth. Rough materials have bumps or ridges on their surface, which can be felt by touching them. Smooth materials lack these bumps. Examples of rough materials are rocks, sandpaper, and bark of a tree. A glass sheet, flower petals, and surface of an apple are some examples of smooth surfaces (Fig. 5.1).

Lustre:
Lustre is the shine of a material. All metals in pure state are shiny and said to possess lustre. This property of metals is widely used for making jewellery and other decorative articles. Materials like gold, silver, and bronze have lustre (Fig. 5.2).

Transparency:
Different materials allow different amounts of light to pass through them depending on a property called transparency. Based on transparency, materials can be of three types: transparent, translucent, and opaque.
Materials that allow all the light to pass through them are called transparent materials (Fig. 5.3). Glass, water, acrylic sheet, and cellophane paper are transparent. Shopkeepers generally prefer to keep items like toffee, biscuit, sweet, etc. in transparent jars so that we can see them easily. Windows are also usually made of glass so that light can pass through and light our rooms.
Materials that allow some light to pass through them are called translucent materials. Oiled paper and coloured glass are translucent materials. Materials that do not allow light to pass through them are called opaque materials. Wood, metal, leaf, stone, and cardboard are opaque materials.

State:
All substances are made up of matter. Matter exists in three states – solid, liquid, and gas.
Table 5.1 Grouping based on the states of matter

Solubility:
Different materials have different solubility in water. Based on their solubility, materials can be soluble, insoluble, miscible, or immiscible.
Solid materials that dissolve in water are said to be soluble in water. For example, common salt and sugar. Solid materials that do not dissolve in water are said to be insoluble in water. For example, sand, wood, stone, chalk powder, and wax. Liquids that dissolve in water are said to be miscible in water. For example, alcohol, vinegar, lemon juice, honey, and glycerine. Liquids that do not dissolve in water are said to be immiscible in water. For example, kerosene, coconut oil, and diesel.
Some gases dissolve in water (e.g., carbon dioxide and oxygen). Oxygen dissolved in water is essential for the survival of aquatic organisms. Soft drinks have carbon dioxide dissolved in them. Gases like nitrogen, hydrogen, and helium are insoluble in water.
Flotation:
Certain materials float on water whereas others sink. This property of a material to float on water is called flotation. Generally, materials like wood, leaf, and feather float on water whereas rock and metal sink.

Attraction towards a magnet:
Materials that are attracted to a magnet are called magnetic materials. This property is called magnetism. Objects made of iron are attracted to a magnet. In addition to iron, nickel and cobalt are also attracted to a magnet.
Conduction of heat:
If you observe the utensils in your kitchen, you will notice that though most of them are made of metals, their handles are made of wood or hard plastic.

Why aren’t the handles made of metal as well? This is because metals get heated whereas materials like plastic and wood do not (Fig. 5.4). It would be difficult to hold the handles made of metal while cooking.
Materials that allow heat to flow through them are called conductors of heat whereas those that do not allow heat to flow through them are called insulators of heat.
Generally, metals are conductors of heat whereas non-metals like wood, plastic, glass, bamboo, air, and paper are insulators of heat.
Conduction of electricity:
We get electricity in our homes through cables and wires. An electric cable consists of a number of metal wires with or without a plastic covering (Fig. 5.5). The metal wires conduct or transmit electricity whereas the plastic covering do not. Materials that conduct electricity are called conductors. Materials that do not conduct electricity are called insulators. For example, metals are conductors of electricity; wood, air, and plastic are insulators.

Transparent Materials that allow light to pass through them are called transparent materials.
Translucent Materials that allow some light to pass through them are called translucent materials.
Opaque Materials that do not allow light to pass through them are called opaque materials.
Miscible Liquids that are soluble in water are said to be miscible in water.
Immiscible Liquids that are insoluble in water are said to be immiscible in water.
Magnetic materials Materials that are attracted by a magnet are called magnetic materials.
Conductors Materials that conduct heat or electricity are called conductors.
Insulators Materials that do not conduct heat or electricity are called insulators.
Materials have different properties that make them useful for making different objects.
An object can be made from different materials.
Different objects can be made from the same material.
Materials can be rough or smooth, transparent or opaque, soluble in water or insoluble, can float on water or sink; can be a conductor or insulators of heat or electricity.