Introduction
Combustion
- A chemical process in which a substance reacts with oxygen to give off heat and light is called combustion.
- The burning of wood is an example of combustion.

Combustible and Non-Combustible Substances
- Substances which easily catch fire are combustible substances, such as paper, coal, wood.
- Substances which do not catch fire readily are non-combustible substances, such as sand, water, glass.
History of Wood and Candle Flame
Fuel
- Any substance, which upon combustion produces a usable amount of energy is known as fuel. For example, fossil fuels, biogas, nuclear energy, etc.
- Fuels can be solid, liquid or gas depending on their state.
- On the basis of their occurrence, it can be either natural or artificial.
Ignition Temp
The lowest temperature at which a combustible substance catches fire when heated in air is called its ignition temperature.
Inflammable Substances
The substances, which have very low ignition temperature and can easily catch fire with a flame are known as inflammable substances. Examples: diesel, LPG, acetone.
Fire
- Fire is the result of a chemical combustion reaction between oxygen and some sort of fuel.
- How long a fire lasts depends on how much fuel and oxygen are available.
Candle Flame
Fire Triangle
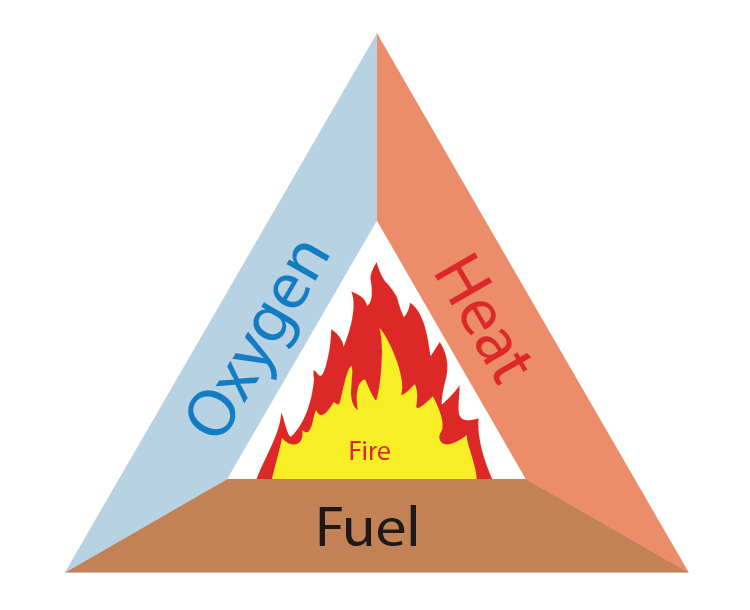
For the generation of fire, we need three things to be present simultaneously:-
- Some sort of fuel or combustible material.
- A heat source to raise the temperature of the fuel to its ignition temperature.
- Enough oxygen to sustain combustion. So, if we remove any one of these resources, the fire can be controlled.
Flame
- Flame is the visible and gaseous part of the fire.
- What we see as the flame is the light energy released due to the combustion of fuel.
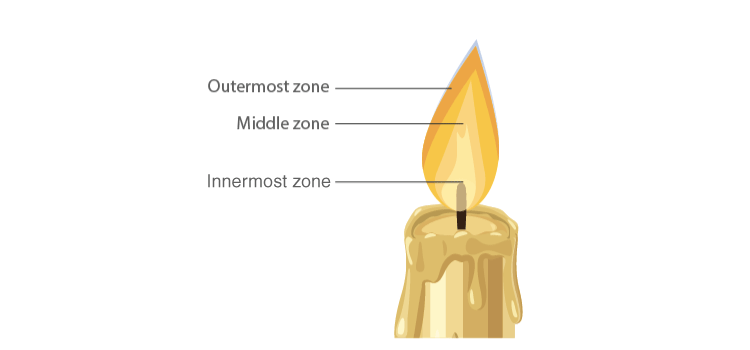
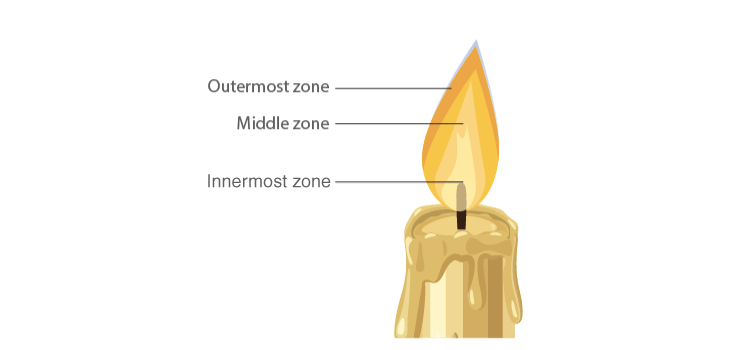
Zones of Candle Flame

Structure of Flame
- The outermost zone is the hottest among all zones and is blue in colour and this is due to complete combustion. It is the non-luminous part of the flame.
- The middle zone of the candle flame is moderately hot and is yellow in colour, and partial combustion of fuel takes place. It is the bright part of the flame.
- The innermost zone of the flame is the least hot and is black in colour. This is due to the presence of unburnt wax vapours.
Smoke
- Smoke is an example of solid (unburnt particles) dispersed in a gas (air).
- The black colour of smoke is due to the presence of unburnt carbon particles in the smoke.
Matchstick
Types of Combustion
- The type of combustion in which heat and light are released in a very short span of time is called rapid combustion. For e.g. combustion of L.P.G.
- The type of combustion in which substances catch fire on their own, without the application of heat is termed as spontaneous combustion. For e.g. forest fires.
Working of a Matchstick
- The main component of the bulb of a matchstick is red phosphorus which turns into white phosphorus on heating.
- White phosphorus spontaneously ignites, thereby increasing the temperature of the wooden stem to the ignition point and the matchstick starts burning.

Fire Extinguisher
Fire Control
Fire can be controlled by removing any or all of the factors of combustion, i.e. fuel, oxygen (air) and ignition temperature (by lowering the temperature).
Fire Extinguisher
- The fire extinguisher is a device used by the fire brigade to control fire.
- The role of the fire extinguishers is to cut off the supply of oxygen or bring down the temperature of the fuel or both.
Calorific Value
Ideal Fuel
- The ideal fuel is cheap, easily available and readily combustible.
- It has high calorific value.
- It does not produce harmful gases or residues that pollute the environment.
Calorific Value and Efficiency of a Fuel
- The amount of heat energy produced on complete combustion of 1 kg of a fuel is called its calorific value. The calorific value of a fuel is expressed in a unit called kilojoule per kg (kJ/kg).
- Efficiency is that proportion of energy released by a fuel combustion process which is converted into useful work.
- Calorific value is directly proportional to its efficiency. If the value is high, it’s efficiency will also be high. If the value is low, it’s efficiency would also be low.
Pollution
Harmful Products from Burning of Fuel
- The burning of fuels like wood, coal and petroleum products releases unburnt carbon particles in the air which causes respiratory problems.
- Incomplete combustion of fuels produces a very poisonous gas called carbon monoxide.
- The burning of fuels releases carbon dioxide in air in the environment which causes global warming.
Unburnt Carbon Particles
- Carbon fuels like wood, coal, candle, petroleum release unburnt carbon particles.
- These fine particles are dangerous pollutants causing respiratory diseases, such as asthma.
CO Emission
- Carbon monoxide is a poisonous gas, which is produced by incomplete combustion of fuels.
- It is dangerous to burn coal in a closed room as the carbon monoxide produced can kill people sleeping in that room.
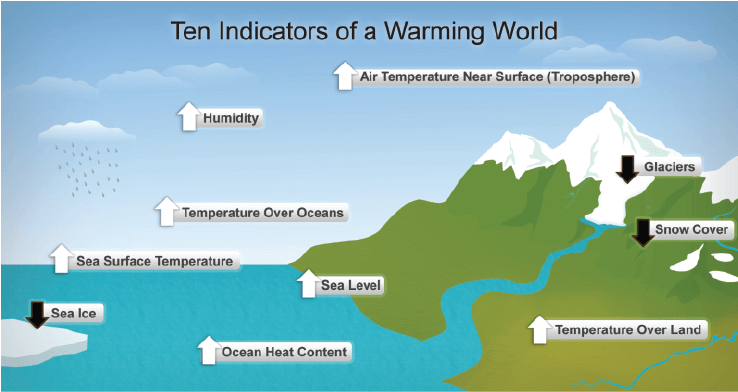
Global Warming
- The rise in the average temperature of the earth’s atmosphere due to the release of carbon dioxide on combustion of fuels is termed as global warming.
- Melting of polar ice-caps or change in the rainfall pattern are the consequences of global warming.
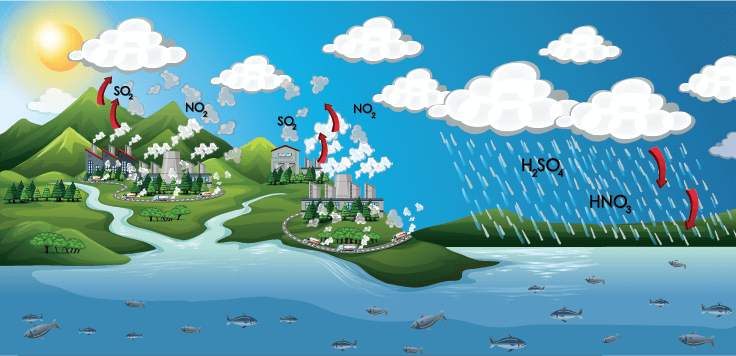
Acid Rain
- Acid rains are caused by emissions of sulphur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acid.
- It has a very harmful effect on plants, land and aquatic animals and infrastructure.