Battery:

Primary battery:
- The reaction in a primary battery occurs only once.
- The battery becomes dead after used onceand cannot be reused
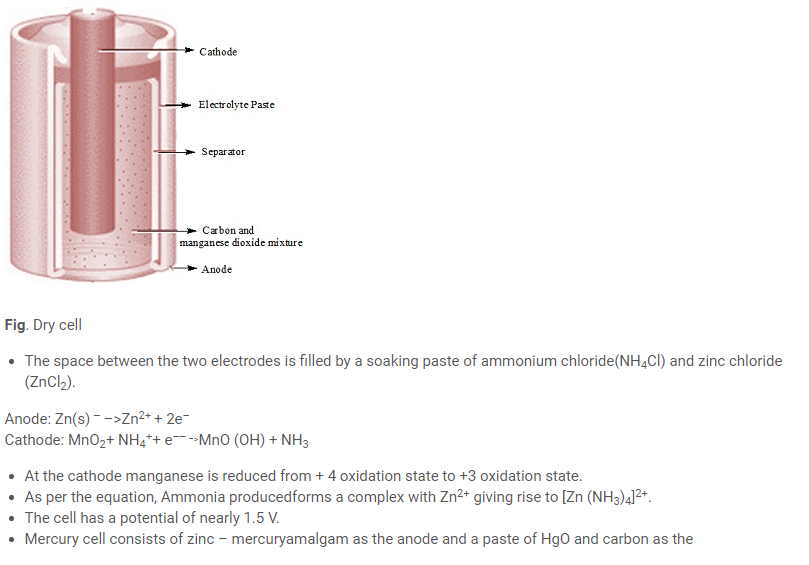
- Example of primary cell is dry cell and mercury cell.
- The dry cell consists of a zincanode and the carbon (graphite) cathode surrounded by powdered manganesedioxide and carbon.

Secondary Battery
- The reaction in a secondary battery occurs many times.
- Once it exhausts it can be recharged and used again.
- They are recharged by passing electric current through it in opposite direction.
- Example of secondary cell is lead and nickel cadmium cell.

Fig. Nickel-Cadmium cell
- It is used extensively in automobiles and invertors.
- The lead cell contains a lead anode and a lead dioxide (PbO2) cathode and sulphuric acid is used as an electrolyte.
Anode: Pb(s) + SO42–(aq) –> PbSO4(s) + 2e–
Cathode: PbO2(s) + SO42–(aq) + 4H+(aq) + 2e– –> PbSO4 (s) + 2H2O (l) - The net cell reaction is:
Pb(s)+PbO2(s)+2H2SO4(aq) –> 2PbSO4(s) + 2H2O(l) - While charging, the reaction within the battery is reversed and PbSO4(s) on anode and cathode is converted into Pb and PbO2, respectively.
- It has a longer life thanlead storage cell and it requires more expenses to
- The net reaction is:
Cd (s)+2Ni(OH)3 (s) –> CdO (s) +2Ni(OH)2 (s) +H2O(l)
Fuel cells
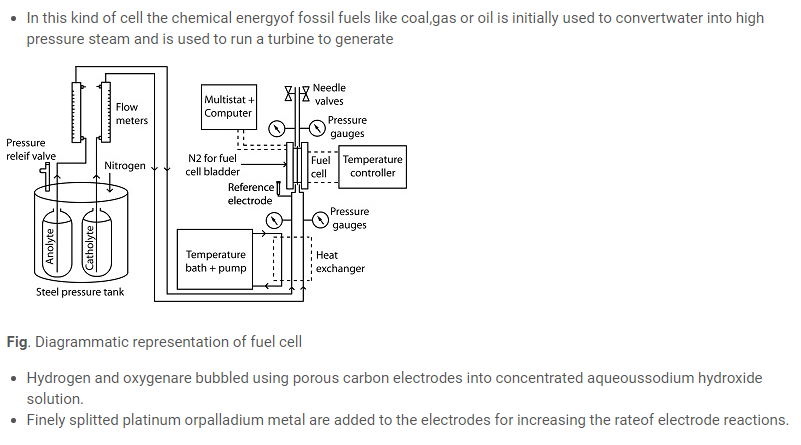
Cathode: O2 (g) + 2H2O (l) + 4e– –> 4OH–(aq)
Anode: 2H2 (g) + 4OH–(aq) ⎯ –> 4H2O(l) + 4e–
- The net reaction is:
2H2(g) + O2(g) →2 H2O(l)
Corrosion
We all must have observed newly bought iron, silver or coper articles appears very shiny but with passage of time they get dull. This is due to the layer of metal oxide that develops on their surface. Rusting of iron, silver jewellery getting tarnished or copper articles getting covered by green layer. Metals react with atmospheric oxygen and produces metal oxides that are basic in nature because they react with water to form bases.
- In case of rusting of iron, the iron reacts with the oxygen present in air and moisture and develops rust (hydrated iron (III) oxide).
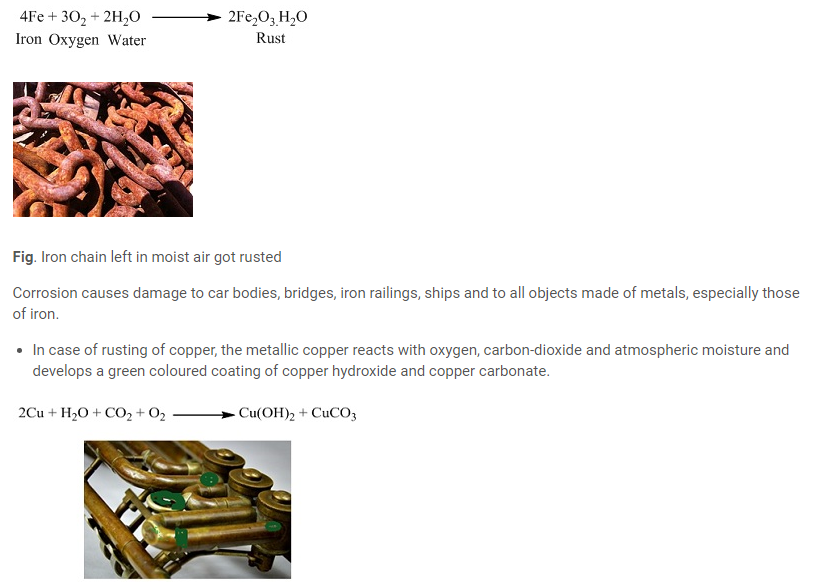
Fig. Copper developing green coloured rust on exposure to moist air
- In case of tarnishing of silver articles, the metallic silver reacts with hydrogen sulphide or sulphur present in air and gets tarnished.
