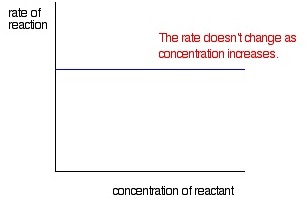
Zeroth order reaction
If the rate of reaction is independent of concentration of the reactant participating in the reaction then the reaction is called Zeroth order reaction.
A –> B
At time t = 0 concentration of A (reactant) is a and B (product) is 0. At time t = t the concentration of A (reactant) is (a-x) and that of B (product) is x.
-d[A]/dt = k0[A]0 => dx/dt = k0(a-x)0
dx/dt = k0
∫ 0x dx =k0 ∫0 xdt
X = k0t
PROBLEM. The decomposition of NH3on platinum surface is zero order reaction. What are the rates of production of N2and H2if k = 2.5 x 10-4mol-1L s-1?
SOLUTION. 2NH3 –> N2 + 3H2
Rate of zero order reaction
-1/2 (d[NH3]/dt) = d[N2]/dt = 1/3 (d[H2]/dt)
-1/2 (d[NH3]/dt) = d[N2]/dt = 1/3 (d[H2]/dt)= k = 2.5 x 10-4 mol L-1 s-1
Rate of production of N2
d[N2]/dt = 2.5 x 10-4 mol L-1 s-1
Rate of production of H2
d[H2]/dt = 3 x 2.5 x 10-4 mol L-1 s-1
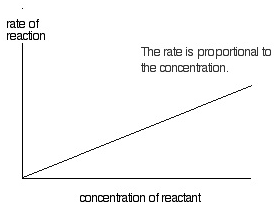
First order reaction
If the rate of reaction depends on the concentration of single reactant participating in chemical reaction raised to the first power then it is called a first order reaction.
A –> B
At time t = 0 concentration of A (reactant) is a and B (product) is 0. At time t = t the concentration of A (reactant) is (a-x) and that of B (product) is x.
-dx/dt ∝ (a-x) = dx/dt = k1(a-x)
∫ 0x dx/(a-x) = k1∫ 0t dt
dx/dt = k0(a-x)0
dx/dt = k0
∫0 x dx = k0∫ 0t dt
ln (a/a-x) = k1t => t = 1/ k1 ln (a/a-x) = 2.303/ k1 log (a/a-x)
k1 = 2.303 log (a/a-x)
PROBLEM. A first order reaction has a rate constant 1.15 10-3s-1. How long will 5 g of this reactant take to reduce to 3 g?
SOLUTION. From the question, we can write down the following information:
Initial amount = 5 g
Final concentration = 3 g
Rate constant = 1.15 10 – 3s – 1
We know that for a 1st order reaction,
t = (2.303/k)log[R0]/[R]
(2.303/1.15X10-3)log[5]/[3]
(2.303/1.15X10-3) X 0.2219 = 444.38 s = 444 s
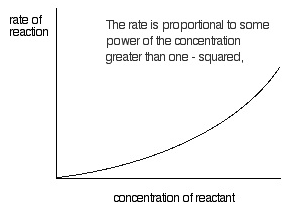
Second order reaction
A reaction with order equal to two is called a second order reaction.
r = k[A]2
or r = k [A][B]
Pseudo order reaction
The reaction that appears to be an nth order reaction but belongs to some different order is called Pseudo order reaction.
For example, a pseudo first order reaction is a chemical reaction between two reactants participating in a chemical reaction and therefore should be a second order reaction. But it resembles to be a first order reaction due to the presence of reactants in negligible quantity.
Let R` + R“ –> P
Rate = k[A]1[B]1
Order of reaction = 2.
Let us consider another reaction,
CH3Br + OH−→ CH3OH+Br−
Rate law for this reaction is
Rate = k [OH−][CH3Br]
Rate = k [OH−][CH3Br] = k(constant)[CH3Br]=k′[CH3Br]
As only the concentration of CH3Br would change during the reaction, the rate would solely depend upon the changes in the CH3Br reaction.