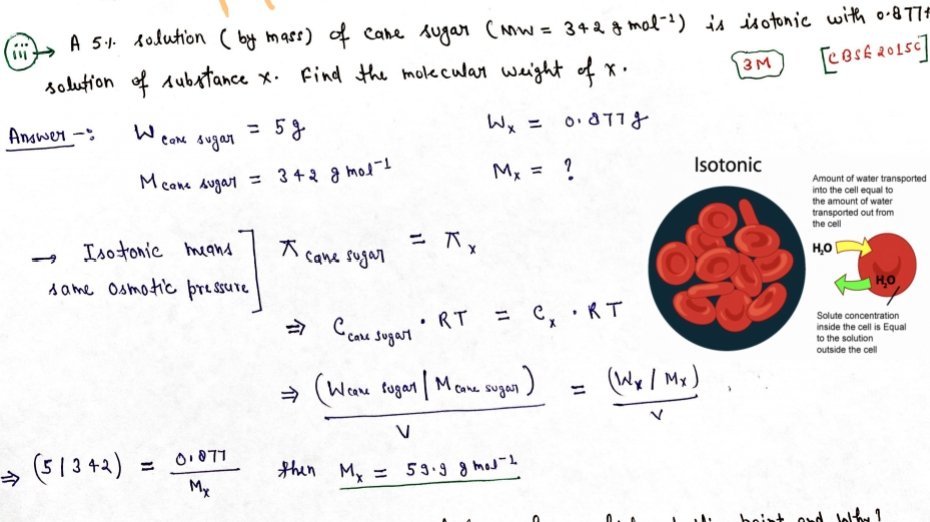
Depression of freezing point
OR
- Freezing point of a substance is the temperature at which the vapour pressure
of the substance in its liquid phase is equal to the vapour pressure in the solid phase. - If the vapour pressure of the solution is equal to the vapour pressure of the pure solid solvent then the solution freezes.
- According to Raoult’s law, addition of non-volatile solute decreases the vapour pressure of the solvent and would be equal to that of solid solvent at lower This decreases the freezing point of thesolvent.
- The decrease in the freezing point = ΔTf = Tf – Tf0. This is known as depression in freezing point.
Tf0 = freezing point of pure solvent
Tf0 = freezing point when non-volatile solute is dissolved
- In a dilute solution the depression of freezing point Tf is directly proportional to molality of the solution. Mathematically,
ΔTf ∝m
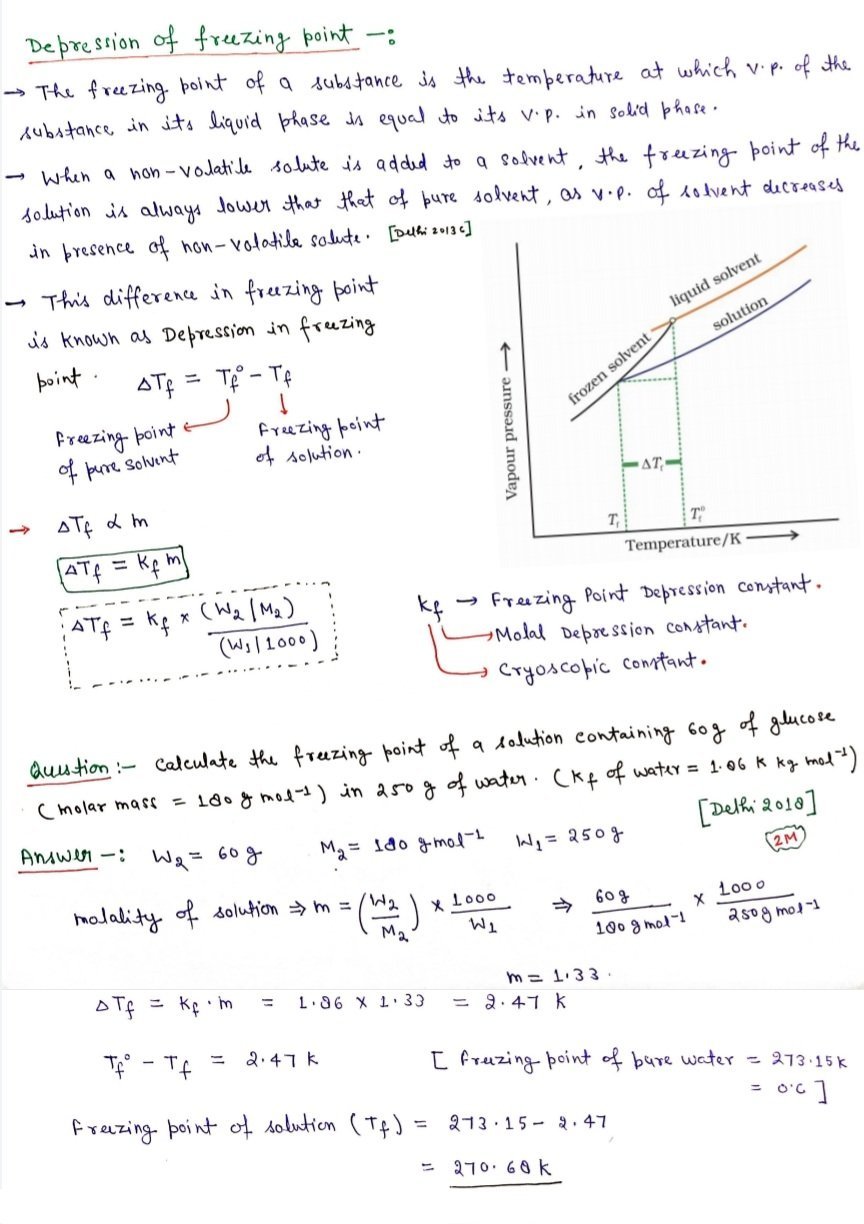
ΔTf = Kf m
Kf = Proportionality constant depending upon the nature of the solvent. This is known as Freezing Point Depression Constant or Molal Depression Constant or Cryoscopic Constant. The unit of Kf is K kg mol-1.
- Let w2 and M2 = Masses and molar masses of solute
w1= Mass of solvent


Osmosis and Osmotic pressure
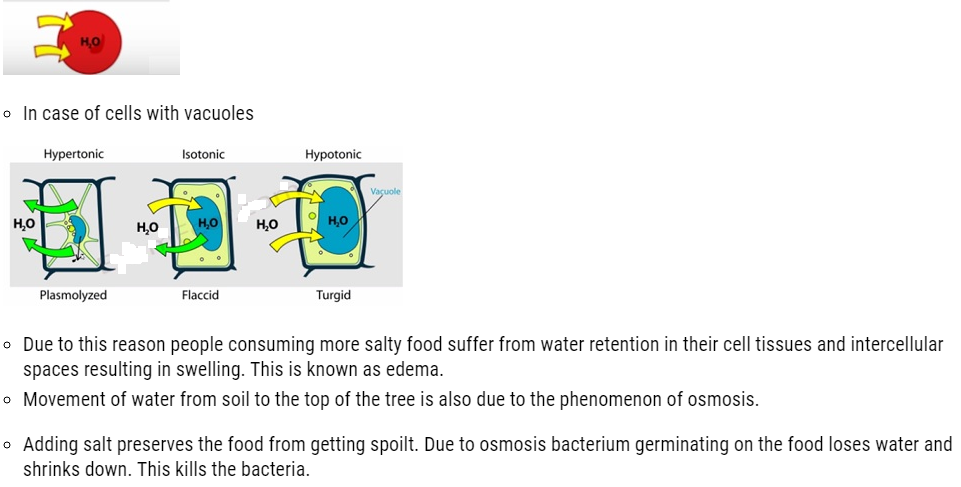
- We have often observed that raw mangoes shrink when kept in salt water (brine solution), flowers remain fresh for longer time when kept in water.
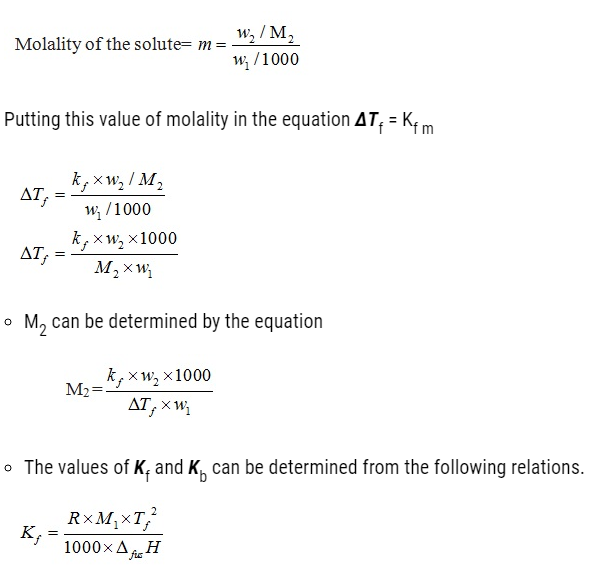
- This is due to the fact that small solvent molecules such as water are capable of passing through the pores present in every cell. These pores allows small solvents like water to pass through but hinders the passing of larger molecules. This membrane possessing such pore is known as semipermeable membranes.
- The process of flow of solvents from the pure solvent to the solution through a semipermeable membrane is termed as osmosis.
- This flow of solvents to the solution continues until the stage of equilibrium is reached.
- Application of extra pressure on the solution can terminate the flow of solvents from the pure solvent to the solution. This pressure is called osmotic pressure of the solution. It is a colligative property that depends upon the concentration of the solution and is basically meant to stop the passage of solvent molecules through a semipermeable membrane into the solution.
- Solvent molecules generally flows from lower concentration to higher concentration. This flow of solvent from dilute solution to the concentrated solution through a semipermeable membrane is due to the phenomenon of osmosis.
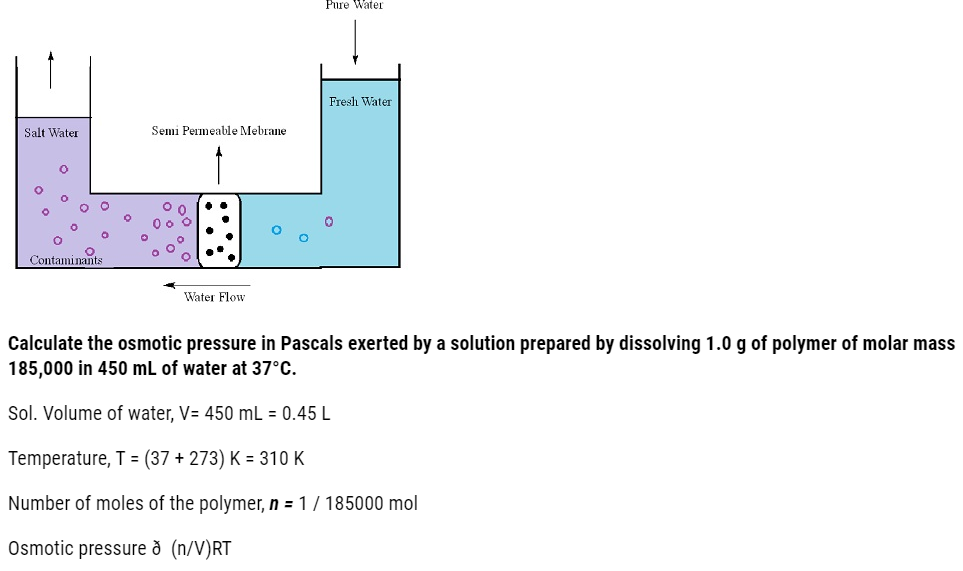
= 1/185000 mol X 1/0.45L X 8.314 X 103 Pa L K-1mol-1 X 310K
= 30.98 Pa = 31 Pa
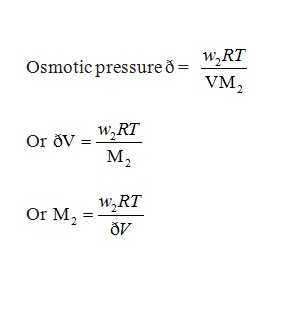
Calculation of Molar Mass from Osmotic Pressure
In dilute solutions the osmotic pressure is proportional to the molarity, C of the solution at a given temperature T. Mathematically,
ð = C R T
R= gas constant
ð = osmotic pressure = (n2/V) RT
V = Volume of a solution in litres
n2 = Moles of solute.
w2 = Grams of solute
M2 = Molar mass
Now, n2 = w2/M2



Use of Reverse osmosis in water purification
Reverse osmosis is extensively used in the desalination of sea water.
A porous film of cellulose acetate is used as a membrane which is permeable to water but impermeable to impurities and ions is placed over a support.
Pressure larger than the osmotic pressure is applied on the sea water solution which allows the passing out of the pure water holding back the bulk of contaminants through the membrane made of polymer.
The measure of pressure required relies upon the salt grouping of the food water. Greater concentration of feed water requires more pressure to defeat the osmotic pressure.
As the water enters the RO membrane under great pressure the water molecules pass through the semi-permeable membrane.
The membrane is impermeable to salts and other contaminants and hence is not allowed to pass through it.
These leftover contaminated water is then discharged through the reject stream and finally drained out or is fed back into the feed water supply. This water will again undergo same procedure and will be purified in some way.

Abnormal molar mass
- Some molecules of solvents with low dielectric constant like molecules of ethanoic acid dimerize into benzene due to the presence of hydrogen bond.
- Association of all the molecules of ethanoic acid into benzene means Tb or Tf for ethanoic acid will be half of the normal value.
- The molar mass calculated on the basis of this ΔTb or ΔTf will be twice the expected molar mass.
- This value of molar mass is either lower or higher compared to expected or normal value and is known as abnormal molar mass.
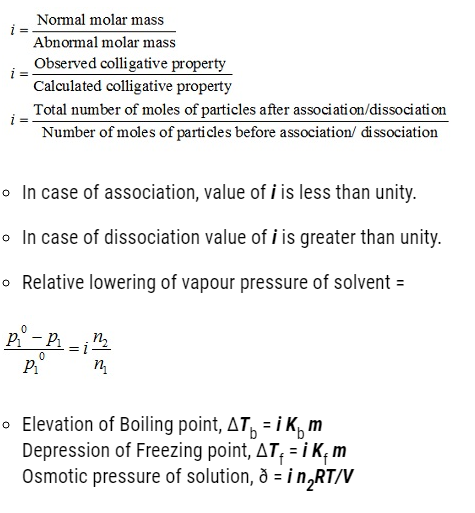
- In 1880 van’t Hoff introduced a factor i and was named as the van’t Hoff factor.
- The van’t Hoff factor account for the degree of dissociation or association.
- The van’t Hoff factor i can be defined as the ratio of Normal molar mass to the abnormal molar mass. Mathematically,
Q.1: The molecular masses of polymers are determined by osmotic pressure method and not by measuring other colligative properties. Give 2 Reason? [Delhi 2019/CBSE 2011C/2010]
Answer:- The osmotic pressure method has the advantage over other colligative properties because:-
- Pressure measurement is arround the room temperature and the molarity of the solution is used instead of molality.
- Its magnitude is large as compared to other colligative properties even for very dilute solutions.
Q. 2: Outer hard shells of two eggs are removed. one of the egg is placed in pure water and the other egg is placed in saturated solution of NaCl. What will be observed and why?
Ans:- The egg placed in water will swell because the concentration of proteins is high inside the egg as compared to water. Therefore, water diffuses through semipermeable membrane of egg and egg swells.
The egg placed in NaCl solution will shrink due to osmosis, water will move out of the egg membrane, thereby, shrinking the egg. [CBSE 2010]
Q. 3: Out of 1M glucose and 2M glucose, which one has a higher boiling point and why?
Answer:- 2M glucose has higher B.P. because more the concentration, more is the elevation in boiling point. [Delhi 2016]