Daniel cell
- The cell that converts the chemical energy liberated as a result of redox reaction to electrical energy is called a Daniel cell.
- It has an electrical potential of 1.1 V.
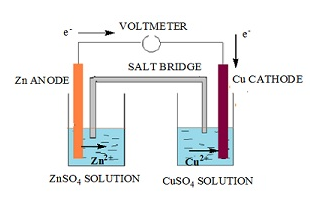
- The setup for Daniel cell is as follows:
- In a beaker a plate of zinc is dipped in a solution of zinc sulfate (ZnSO4).
- In another beaker a plate of copper is dipped in a solution of copper (II) sulfate in another container. These plates of metal are called the electrodes of the cell.
- These electrodes behave as terminal to hold the electrons.
- The two electrodes are connected via wire.
- A salt bridge is placed between the two beakers. This provides a path for the movement of ions from one beaker to the other in order to maintain electrical neutrality.
- Zinc electrode gets oxidized and hence releases electrons that flow through the wire towards the copper electrode.
- The copper (II) sulfate solution releases copper ions Cu2+.
- At the anode:
Oxidation —————- loss of electrons.
Zn –> Zn2+ + 2e–At cathode,
Reduction ————-gain of electrons.
Cu2+ + 2e– –> Cu
- Zinc atoms being more reactive have a greater tendency to lose electrons than that of copper.
The electrons in this cell moves from zinc anode to copper cathode through the wire connecting the two electrodes in the external circuit - A bulb placed within this circuit will glow and a voltmeter connected within this circuit will show deflection.
- The net reaction of this cell is the sum of two half-cell reactions.
Zn(s) + Cu2+ (aq) –> Zn2+ (aq) + Cu(s)
In a Daniel cell a salt bridge is placed between the two beakers containing a solution of zinc sulfate (ZnSO4) and a solution of copper (II) sulfate respectively. This provides a path for the movement of ions from one beaker to the other in order to maintain electrical neutrality.
Shorts Notes on Daniell Cell:-

Salt Bridge
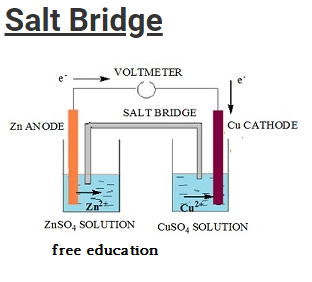
- Salt bridge is an inverted U-tube like structure.
- The tube is filled with concentrated solution of an inert electrolyte.
- The electrolyte does not undergo any sort of chemical reaction nor does it reacts with concentrated solution in the two half cells.
- Most of the time salts like KCl, KNO3, NH4NO3 are used as electrolyte.
- Salt bridge is constructed by filing the U-tube with a mixture prepared by mixing agar-agar or gelatin with a hot concentrated solution of the electrolyte.
- The apparatus is then cooled and consequently the solution fixes within the U-tube in the form of gel.
- This prevents the intermixing of fluids.
- The either ends of the tube are then sealed using cotton wool in order to reduce diffusion.
- The main advantage of suing this bridge is that it prevents the arousal of potential difference (also called liquid junction potential) when two solutions are in contact with each other.
- It completes the electrical circuit as it connects the electrolytes in the two half cells.
- It avoids the chances of diffusion of solutions between the half cells.
- It maintains electrical neutrality by providing a path for the movement of ions.

Measurement of electrode potential in a cell
- The electrode is connected to a standard hydrogen electrode (SHE) to constitute a cell.
- The electrode forming the negative terminal of the cell is allotted negative value of electrode potential whereas the electrode forming the positive terminal of the cell is allotted a positive value of electrode potential.
- The potential difference developed between the two terminals is measured using a potentiometer.
- The direction of the flow of electric current in the external circuit is identified using a galvanometer.
- This enables us to identify the positive and the negative terminal of the set up as the current flows from positive terminal to negative terminal.
- This in turn will help us mark the anode and the cathode electrodes due to the fact that the electrons will flow from anode to cathode.
Ecell = Ecathode – Eanode
- In case of Daniel cell
At the anode:
Oxidation —————- loss of electrons.
Zn –> Zn2+ + 2e–
At cathode,
Reduction ————-gain of electrons.
Cu2+ + 2e– –> Cu
The net reaction of this cell is the sum of two half-cell reactions.
Zn(s) + Cu2+ (aq) –> Zn2+ (aq) + Cu(s)
Emf of the cell = Ecell = Ecathode – Eanode
= 0.34 V – (- 0.76) V = 1.10 V
(Measured Emf of Cu is 0.34 V and that of Zn is 0.76 V).
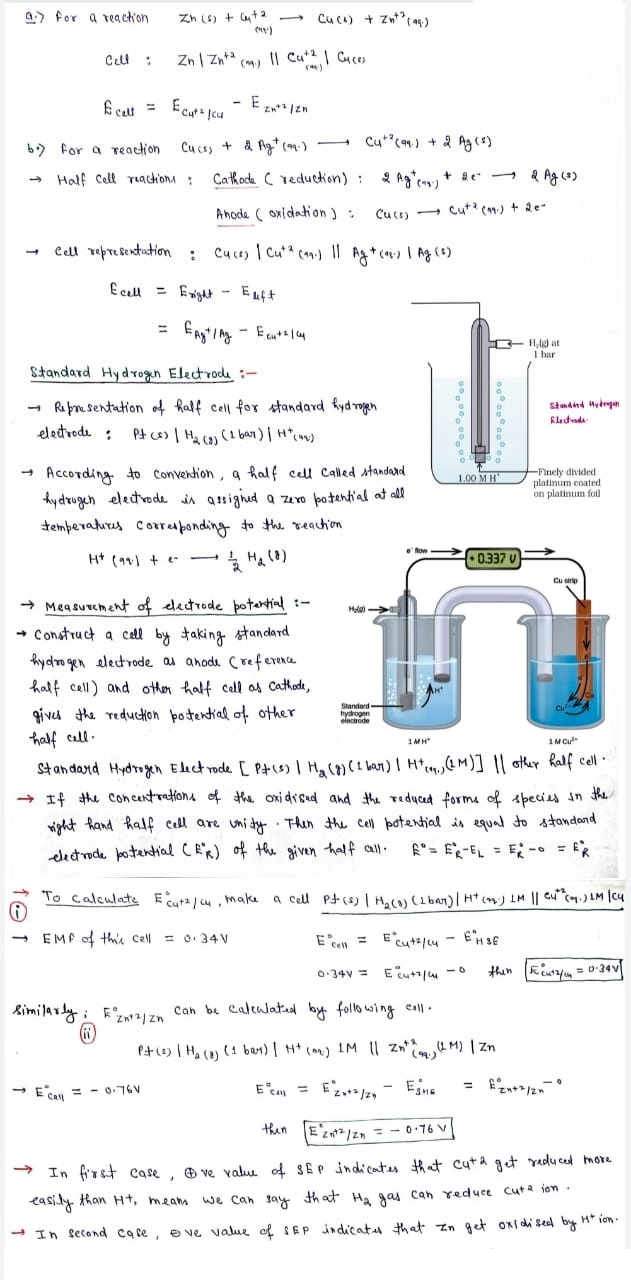
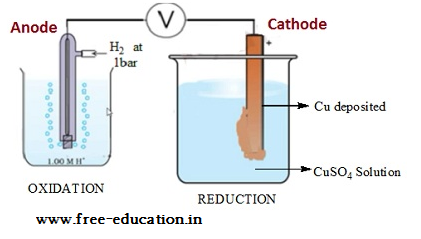
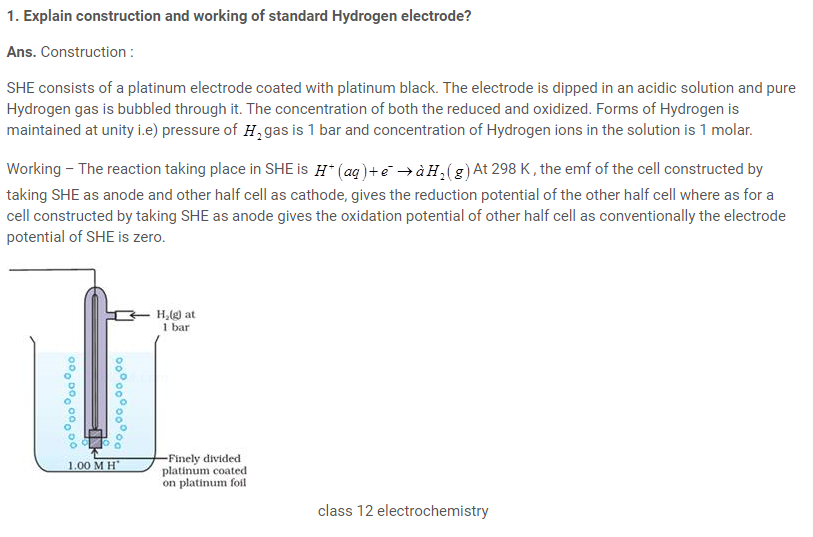
Standard hydrogen electrode
- The electrode is connected to a standard hydrogen electrode (SHE) to constitute a cell
- It consists of a platinum electrodecoated with a layer of platinum black.
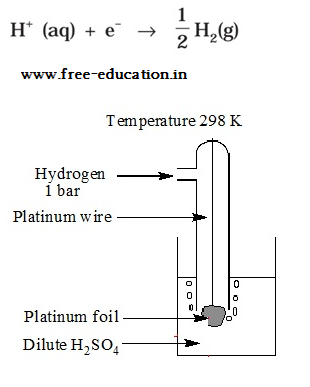
- The electrode is immersed in an acidicsolution and the pure hydrogen gas is bubbled through it.
- Theconcentration of the reduced form and the oxidized form of hydrogen issustained at unity with following conditions:
- Pressure of hydrogen gas = 1 bar
- Concentration of hydrogen ion in the solution = 1 molar
Ecell = Ecathode – Eanode
Ecell = Ecathode – 0 = Ecathode
- The measured Emf of the cell:
Pt| H2 (1 bar)| H+ (1M) || Cu2+ (1M)| Cu is 0.34 V.
The positive value of the standard electrode potential signifies the easy reduction of Cu2+ ions than H+ ions.
- The measured Emf of the cell
Pt| H2 (1 bar)| H+ (1M) || Zn2+ (1M)| Zn is -0.76 V.
The negative value of the standard electrode potential signifies that the hydrogen ions oxidizes the zinc (or it can be said that zinc can reduce hydrogen ions).
- An electrode with standard electrode potential greater than zero is stable in its reduced form compared to hydrogen gas.
- Whereas an electrode with negative standard electrode potential is less stable in its reduced form compared to hydrogen gas.
- This decreases the standard electrode potential which in turn decreases the oxidizing power of the specific electrode on the left and increases the reducing power of the electrode to the right of the reaction.


Question 9.
The chemistry of corrosion of iron is essentially an electrochemical phenomenon. Explain the reactions occurring during the corrosion of iron in the atmosphere. (Delhi 2011)
Answer:
The mechanism of corrosion is explained on the basis of electrochemical theory. By taking example of rusting of iron, we Refer tothe formation of small electrochemical cells on the surface of iron.
The redox reaction involves
At anode : Fe(S) → Fe2+ (aq) + 2e–
At cathode : H2O + CO2 ⇌ H2CO3 (Carbonic acid)
H2CO3 ⇌2H+ + CO22-
H2O ⇌ H+ + OH–
H+ + e– → H
4H + O2 → 2H2O
Then net resultant Redox reaction is
2Fe(s) + O2 (g) + 4H+ → 2Fe2+ + 2H2O
Question 11.
Two half-reactions of an electrochemical cell are given below :
MnO–4 (aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I), E° = 1.51 V
Sn2+ (aq) → Sn4+ (aq) + 2e–, E° = + 0.15 V.
Construct the redox equation from the standard potential of the cell and predict if the reaction is reactant favoured or product favoured. (Delhi 2011)
Answer:
The reactions can be represented at anode and at cathode in the following ways :
At anode (oxidation) :
Sn2+ → = Sn4+ (aq) + 2e– ] × 5 E° = + 0.15 V
At cathode (reduction) :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I)] × 2 E° = + 1.51 V
The Net R × M = 2MnO–4(aq) + 16H+ + 5Sn2+ → 2Mn2+ + 5Sn4+ + 8H2O
Now E°cell = E°cathode – E°anode
= 1.51 – 0.15 = + 1.36 V
∴ Positive value of E°cell favours formation of product.
Question 48.
Why an electrochemical cell stop working after some time? The reduction potential of an electrode depends upon the concentration of solution with which it is in contact. (All India 2017)
Answer:
As the cell works, the concentration of reactants decrease. Then according to Le chatelier’s pri¬nciple it will shift the equilibrium in backward direction. On the other hand if the concentration is more on the reactant side then it will shift the equilibrium in forward direction. When cell works concentration in anodic compartment in cathodic compartment decreases and hence E° cathode will decrease. Now EMF of cell is
E0cell = E0cathode – E0anode
A decrease in E°cathode and a corresponding increase in E°anode will mean that EMF of the cell will decrease and will ultimately become zero i.e., cell slops working after some time.


NCERT EXERCISES
3.1. Arrange the following metals in the order in which they displace each other from their salts.
Al, Cu, Fe, Mg and Zn
Sol: Mg, Al, Zn, Fe, Cu.
3.2. Given the standard electrode potentials, K+/K=-2. 93 V, Ag+/Ag = 0.80 V, Hg2+/Hg =0.79V, Mg2+/Mg=-2.37V, Cr3+/Cr=0.74V.
Arrange these metals in their increasing order of reducing power.
Sol: Higher the oxidation potential more easily it is oxidized and hence greater is the reducing power. Thus, increasing order of reducing power will be Ag<Hg<Cr<Mg<K.
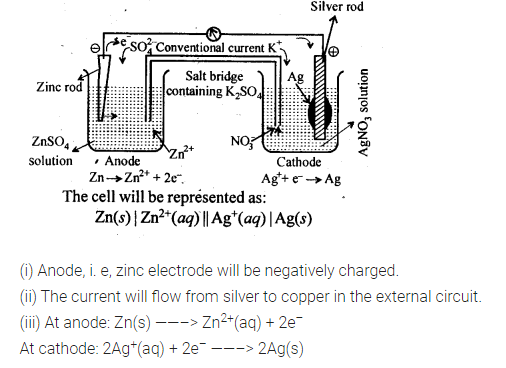
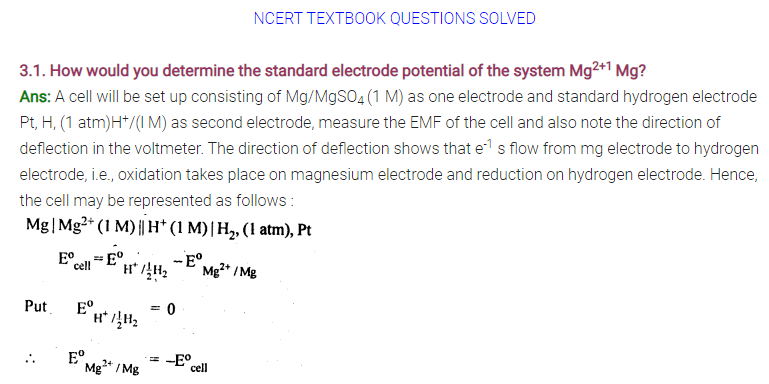
3.3. Depict the galvanic cell in which the reaction
Zn(s) + 2Ag+(aq) —-> 7M2+(aq) + 2Ag (s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Sol. The set-up will be similar to as shown below,