Electrical Properties of soilds
Solids exhibit electrical conductivities that extend from 27 orders of magnitude ranging from 10–20 to 107 ohm–1 m–1.
Conductors:
- Solids with conductivities ranging between 104 to 107 ohm–1m–1 are conductors.
- Metals have conductivities in the order of 107 ohm–1m–1 are good conductors.
- For example, Iron, Copper, Aluminum.

A man touching the electric pole with a metal rod will get an electric shock because metal rod is a conductor whereas a man touching the same with a wooden plank will be safe because wood is an insulator.
- Semiconductors:
- Solids with conductivities in the intermediate range from 10–6 to 104 ohm–1 m–1.
- For example, Gallium, Germanium, Silicon

- Metals conduct electricity both in solid state as well as molten state.
- The conductivity of metals depends upon the number of valence electrons available per atom.
- The atomic orbitals of metal atoms form molecular orbitals that are close in energy to each other as to form a band.
- Partial filling or overlapping with a higher energy unoccupied conduction band enables the electrons to flow easily under an applied electric field.
- This results in conductivity of metals.
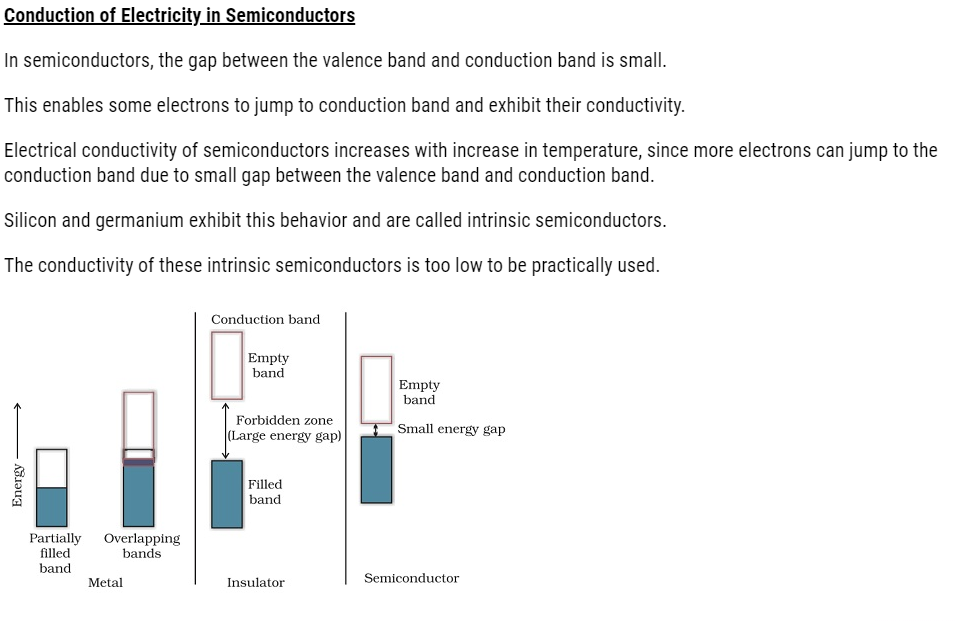
If the gap between valence band and the conduction band is large, electrons cannot jump to it and such a substance has very low conductivity that makes it behave like an insulator

Number of cation vacancies in lattice of NaCl = Number of divalent Sr2+ ions added.
Concentration of cation vacancy on doping with 10-3 mol% of SrCl2. = 10-3 mol% = 10-3 / 100 = 10-5 mol.
Number of Sr2+ ion in 10-5 mol = 10-5 x 6.023 x 1023 = 6.023 x 1018
Hence number of cationic vacancies = 6.023 x 1018
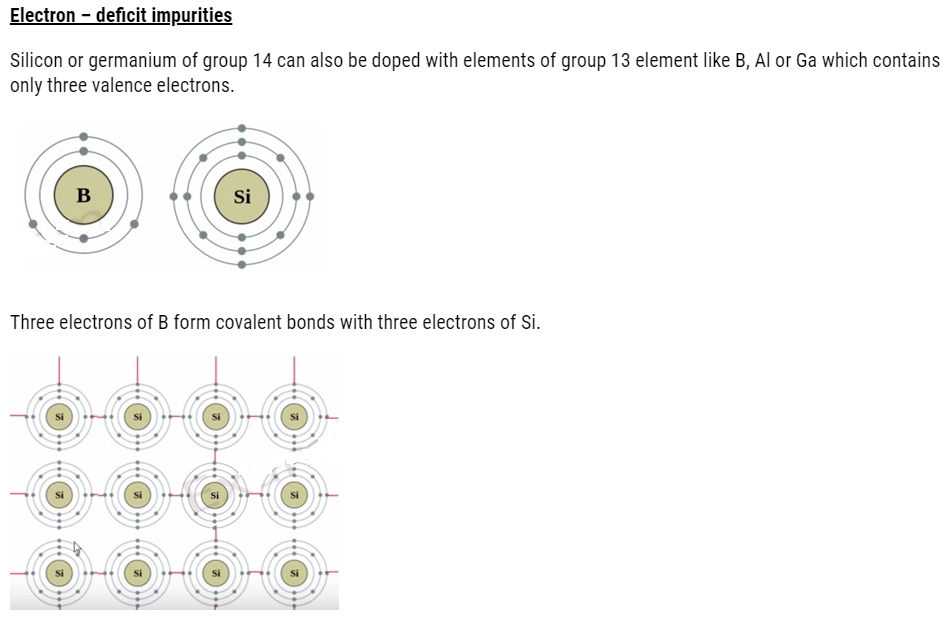
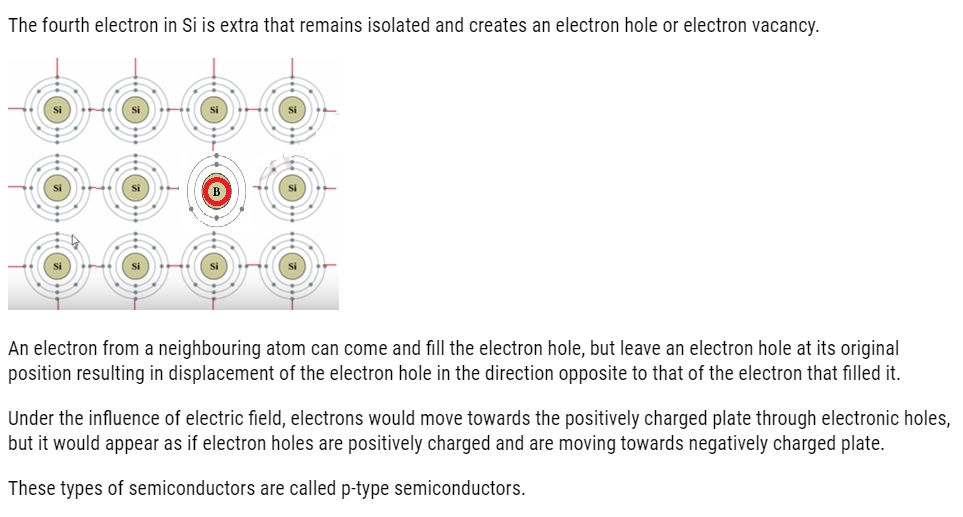
Electron – rich impurities
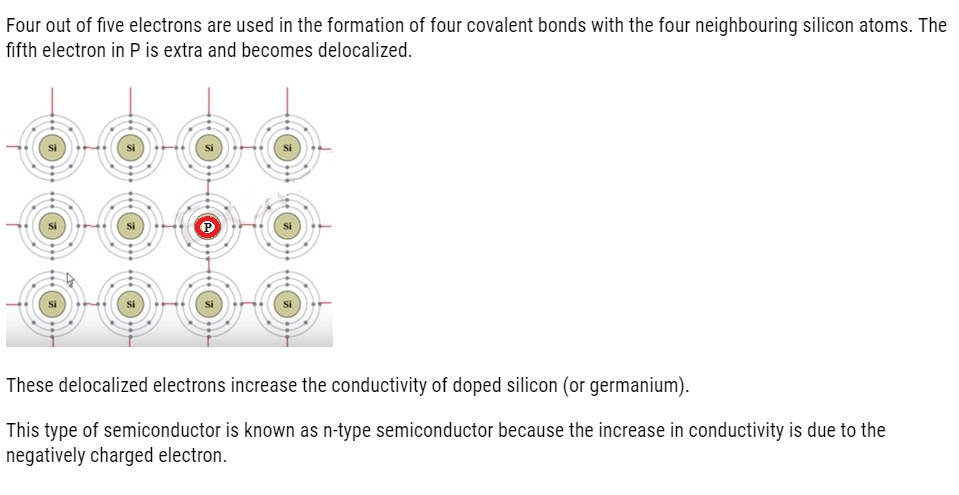
In a periodic table Silicon and germanium belongs to group 14 with four valence electrons each. In their crystals each atom forms four covalent bonds with surrounding atom.
When doped with a group 15 element like P or As, which contains five valence electrons,

Applications of n-type and p-type semiconductors
- n-type and p-type semiconductors finds a great use in manufacturing electronic components.
- Diode is a combination of n-type and p-type semiconductors extensively used as a rectifier.
- Transistors are manufactured by keeping a layer of one type of semiconductor between two layers of another type of semiconductor.
- npn and pnp type of transistors are used to detect or amplify radio or audio signals.
- The solar cell is an efficient photo-diode used for conversion of light energy into electrical energy.
- Gallium arsenide (GaAs) semiconductors have very fast response and have transformed the design of semiconductor devices.
- Transition metal oxides show marked differences in electrical properties.
- TiO, CrO2 and ReO3 behave like metals.
- Rhenium oxide, ReO3 resembles metallic copper in terms of its conductivity and appearance.
- Certain other oxides like VO, VO2, VO3 and TiO3 exhibit metallic or insulating properties depending on temperature.
1.23. A group 14 element is to be converted into n-type semiconductor by doping it with a suitable impurity. To which group should this impurity belong? (NCERT Book)
Ans: Impurity from group 15 should be added to get n-type semiconductor.
Question 17. ( The Solid State NCERT Solution )
What are semi-conductors? Describe the two main types of semiconductors and contrast their conduction mechanisms.
Solution:
Semi-conductors are the substances whose conductivity lies in between those of conductors and insulators. The two
main types of semiconductors are n-type and p-type.
(i) n-type semiconductor: When a silicon or germanium crystal is doped with group 15 element like P or As, the dopant atom forms four covalent bonds like Si or Ge atom but the fifth electron, not used in bonding, becomes delocalised and continues its share towards electrical conduction. Thus silicon or germanium doped with P or As is called H-type semiconductor, a-indicative of negative since it is the electron that conducts electricity.



(i) In metals, conductivity strongly depends upon the number of valence electrons available in an atom. The atomic orbitals of metal atoms form molecular orbitals which are so close in energy to each other, as to form a band. If this band is partially filled or it overlaps with the higher energy unoccupied conduction band, then electrons can flow easily under an applied electric field and the metal behaves as a conductor.
If the gap between valence band and next higher unoccupied conduction band is large, electrons cannot jump into it and such a substance behaves as insulator.
(ii) If the gap between the valence band and conduction band is small, some electrons may jump from valence band to the conduction band. Such a substance shows some conductivity and it behaves as a semiconductor. Electrical conductivity of semiconductors increases with increase in temperature, since more electrons can jump to the conduction band. Silicon and germanium show this type of behaviour and are called intrinsic semiconductors. Conductors have no forbidden band.