About Lesson
Strong electrolytes
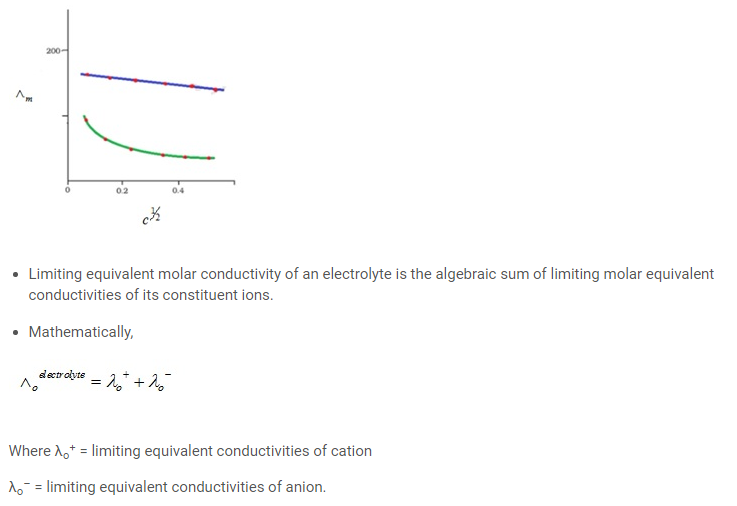
- A solute or substances that completely ionize or dissociates in a solution are known as strong electrolyte. These ions are good conductors of electricity in the solution.
- For example, HCl, HBr, HI, HNO3, NaOH, KOH, etc.
- For strong electrolytes, Λm increases slowly with dilution and can berepresented by the equation:

Kohlrausch law of independent migration of ions
- The Kohlrausch law of independent migration of ions states that limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte.
Weak electrolytes
- A substance which forms ions in an aqueous solution do not dissociate completely at moderate concentrations is known as weak electrolyte.
- For example, HC2H3O2 (acetic acid), H2CO3 (carbonic acid), etc.
- The conductance of the solution increases with dilution of solution.
- The interionic forces of attraction are not strong at a low concentration.
- The slope for Λm vs c1/2 is not linear even at a lower concentrations.

- These electrolytes have lower degree of dissociation at higher concentrations.
- The value of Λm changes with dilution due to increase in the degree of dissociation.
- Ëm increases sharply on dilution exclusively at lower concentrations.
- At infinite dilutionwhen concentration approaches to zero, the electrolyte dissociates completely. But at lower concentration the conductivity of a solution is low to an extent that cannot be even measured.