Electrolysis
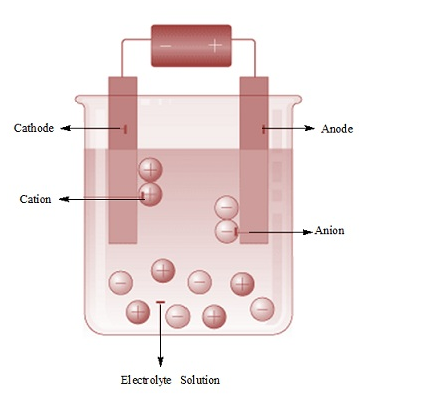
Fig. Electrolysis
- Two copper strips are dipped in an aqueous solution ofcopper sulphate (CuSO4).
- A DC voltage is applied to both the electrodes.
- Cu 2+ ions discharge at the cathode
Cu2+(aq) + 2e– –> Cu (s)
- Copper metal is deposited on the cathode and copper is converted into Cu2+ ions at the anode by the following reaction:
Cu –> Cu2+(aq) + 2e–
- The impure copper is the anode that dissolves on passing current whereas the pure copper gets deposited at the cathode. Metals like Na, Mg, Al, etc. are produced using electrochemical reduction of their respective cations.
Faraday’s Laws of Electrolysis
- Michael Faraday described the quantitative aspects of electrolysis and came forward with two laws of electrolysis:
- 1st Law: The amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the quantity of electricity passed through the electrolyte (solution or melt).
- 2nd Law: The amounts of different substances liberated by the same quantity of electricity passing through the electrolytic solution are proportional to their chemical equivalent weights
- Mathematically, Atomic Mass of Metal ÷ Number of electrons required to reduce the cation.
Q = It
where Q is in coloumb
I is in ampere and
t is in second.
- This quantity of electricity is known as Faraday and is represented by the symbol F.

- In the above example application of more current results in the deposition of more amount of copper from the anode to the cathode.
PROBLEM:
IF A CURRENT OF 0.5 AMPERE FLOWS THROUGH A METALLIC WIRE FOR 2 HOURS, THEN HOW MANY ELECTRONS WOULD FLOW THROUGH THE WIRE?
Solution:
I = 0.5 A
t = 2 hours = 7200 s
By using the formula, Q = It
= 0.5 A × 7200 s
= 3600 C
We know that 96487C = 6.023 X 1023 number of electrons.

Products of Electrolysis
- The product of electrolysis produced depends on the nature of material being that is being electrolyzed as well as the type of electrodes that is being used.
- An inert electrode e.g., platinum or gold does not participate in chemicalreaction and acts as a source or sink for electrons.
- Whereas a reactive electrode participates in the electrode reaction.
- It also depends on the different oxidizingas well as reducing species that are present in the electrolytic cell and their standardelectrode potentials.
- Electrolysis of molten NaCl results in the production of sodium metal and Cl2

Problem:
Given the standard electrode potentials,
K+/ K = – 2.93V,
Ag+/ Ag = 0.80V,
Hg2+ / Hg = 0.79V
Mg2+ / Mg = – 2.37 V,
Cr3+ / Cr = – 0.74V
Arrange these metals in their increasing order of reducing power.
Solution:
Lower the reduction potential leads to higher reducing power. The given standard electrode potentials increases in the following order:
K+/ K < Mg2+ / Mg < Cr3+ / Cr < Hg2+ / Hg < Ag+ / Ag.
Hence, reducing power of the given metals increases in the following order:
Ag < Hg < Cr < Mg < K