Classification of crystalline Solid
[Based on inter-particle forces]
- Molecular Solid
- Ionic Solid
- Covalent/Network Solids
- Metallic Solid
1. Molecular Solids
Solid composed of molecules as constituent particles. These solids can further be categorized into following types:
(i) Non polar Molecular Solids: This solid is formed by either atoms (Ar,He) or molecules formed by non-polar covalent bonds (H2,Cl2).
- They are composed of either atom.
- H2, Cl2 and I2.are some of the typical example.
- In non-polar Molecular solids the comprised atoms or molecules are held together by weak dispersion forces or London forces.
- They are soft and non-conductors of electricity.
- They have low melting points and usually exist in liquid or gaseous state at room temperature and pressure.

(ii) Polar molecular solids:-
- The molecules like HCl, SO2, are formed by polar covalent bonds.
- In polar Molecular solids the comprised atoms or molecules are held together by stronger dipole-dipole interactions.
- They are soft and non-conductors of electricity.
- The melting points of these solids are higher than those of non-polar molecular solids ( Melting points : PMS > NPMS)
- usually exist in liquid or gaseous state at room temperature and pressure.
- Molecules like SO2 and solid NH3 are some examples of such solids.

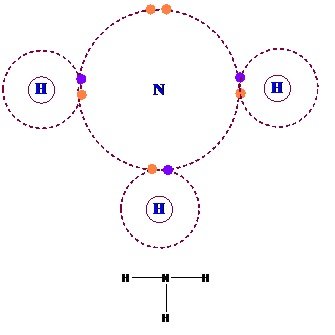
(iii) Hydrogen bonded molecular solids:-
- Hydrogen Bonded Molecular Solids contain polar covalent bonds between H and F, O or N atoms.
- Strong hydrogen bonding binds molecules of such solids like H2O (ice).
- They are non-conductors of electricity and are volatile liquids or soft solids under room temperature and pressure.

2. Ionic Solids
- The constituent particles of these solids are ions.
- The solid is composed of three dimensional arrangements of cations and anions that are bonded by strong coulombic (electrostatic) forces.
- These solids are hard and brittle in nature and have high melting and boiling points.
- They are electrical insulators in solid state due to the absence of movement of free electrons but are good conductors in molten state due to the movement of ions.

3.Metallic Solids
- In these solids positive ions is surrounded by mobile free electrons and are evenly spread all over the crystal.
- Each metal atom donates one or more electrons to the group of mobile electrons which increases the electrical and thermal conductivity of the metallic elements.
- Application of electric field makes these electrons flow through the linkage of positive ions.
- Whereas application of heat to one portion of a metal makes the thermal energy spread uniformly throughout by free electrons.
- Presence of free electrons in metals makes them lustrous, malleable and ductile. For example, Cr, Fe etc.
Note:- Metals are collection +ve ions surrounded by and held together by a sea of free electrons. Example -> Na metal crystal.

4. Covalent solids
- Crystalline solids of non-metals comprises of covalent bonds between adjacent atoms that are strong and directional in nature due to which atoms are held very strongly at their positions all over the crystal.

Fig. Covalent bonding between Carbon and hydrogen
- They are also called giant molecules.
- These solids are very hard and brittle.
- They have extremely high melting points and may decompose before melting.
- They are insulators and do not conduct electricity.
- Diamond and silicon carbide are typical examples of such solids but Graphite is an exception as it is soft and a good conductor of electricity.


Q. Define Metallic Solid with Example ? (CBSE 2016/2014C/ Delhi 2010)
Ans. Metals are collection of +ve ions surrounded by and held together by a sea of free electrons. Example -> Na metal crystal.
Q. What makes graphite soft and a good conductor of electricity?
- Graphite is a covalent solid that act as a good conductor of electricity and is soft.
- The carbon atoms of graphite are prescribed in different layers and are covalently bonded to three of its surrounding atoms in the same layer.
- The fourth valence electron of each atom is present between different layers and is free to move which makes graphite a good conductor of electricity.
- The characteristic of sliding between different layers makes graphite a soft solid and a good solid lubricant.
Q 1.6. Classify the following solids in different categories based on the nature of the intermolecular forces: sodium sulphate, copper, benzene, urea, ammonia, water, zinc sulphide, diamond, rubedium, argon, silicon carbide. (NCERT Book)
Ans: Ionic, metallic, molecular, molecular, molecular (hydrogen-bonded), molecular (hydrogen-bonded), ionic, covalent, metallic, molecular, covalent (network).
Q. 1.7. Solid A is a very hard electrical insulator in. solid as well as in molten state and melts at extremely high temperature. What type of solid is it? (NCERT Book)
Ans: It is a covalent or network solid.
Q 1.8. Why are ionic solids conducting in the molten state and not in the solid-state?
Ans: In the ionic solids, the electrical conductivity is due to the movement of the ions. Since the ionic mobility is negligible in the solid state, these are non-conducting in this state. Upon melting, the ions present acquire some mobility. Therefore, the ionic solids become conducting
Q 1.9. What type of solids are electrical conductors, malleable and ductile?
Ans: Metallic solids
Q. (NCERT Book)
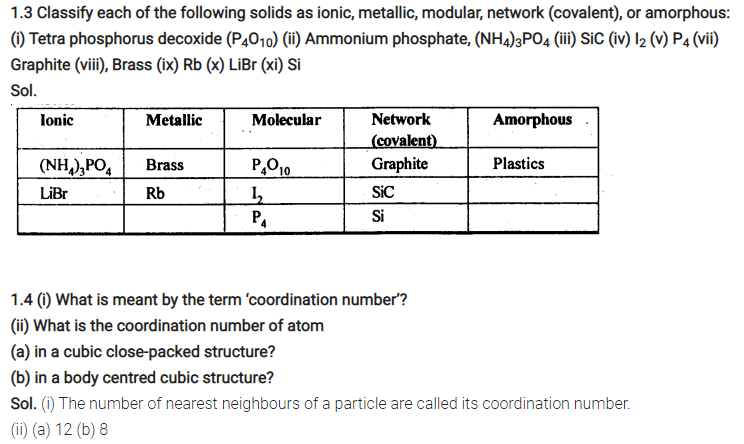
Q. 1.6 ‘Stability of a crystal is reflected in the magnitude of its melting points’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules? (NCERT Book)
Sol. Higher the melting point, greater are the forces holding the constituent particles together and thus greater is the stability of a crystal. Melting points of given substances are following. Water = 273 K, Ethyl alcohol = 155.7 K, Diethylether = 156.8 K, Methane = 90.5 K.
The intermoleoilar forces present in case of water and ethyl alcohol are mainly due to the hydrogen bonding which is responsible for their high melting points. Hydrogen bonding is stronger in case of water than ethyl alcohol and hence water has higher melting point then ethyl alcohol. Dipole-dipole interactions are present in case of diethylether. The only forces present in case of methane is the weak van der Waal’s forces (or London dispersion forces).
Q. 1.9 Explain: (NCERT Book)
(i) The basis of similarities and differences between metallic and ionic crystals.
(ii) Ionic solids are hard and brittle.
Sol. (i) Metallic and ionic crystals
Similarities:
(a) There is electrostatic force of attraction in both metallic and ionic crystals.
(b) Both have high melting points.
(c) Bonds are non-directional in both the cases.
Differences:
(a) Ionic crystals are bad conductors of electricity in solids state as ions are not free to move. They can conduct electricity only in die molten state or in aqueous solution. Metallic crystals are good conductors of electricity in solid state as electrons are free to move.
(b) Ionic bond is strong due to strong electrostatic forces of attraction.
Metallic bond may be strong or weak depending upon the number of valence electrons and the size of the kernels.
(ii) Ionic solids are hard and brittle.Ionic solids are hard due to the presence of strong electrostatic forces of attraction. The brittleness in ionic crystals is due to the non- directional bonds in them.