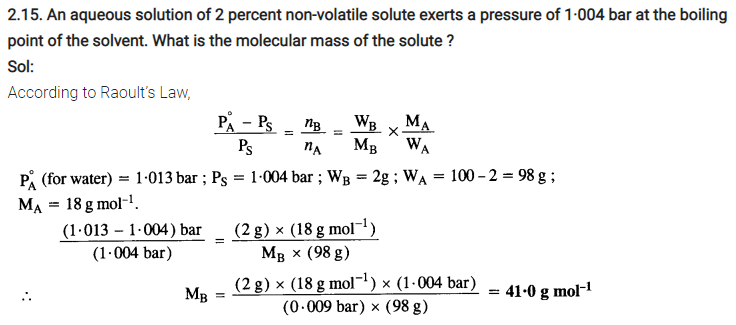Properties of solution affected by vapour pressure
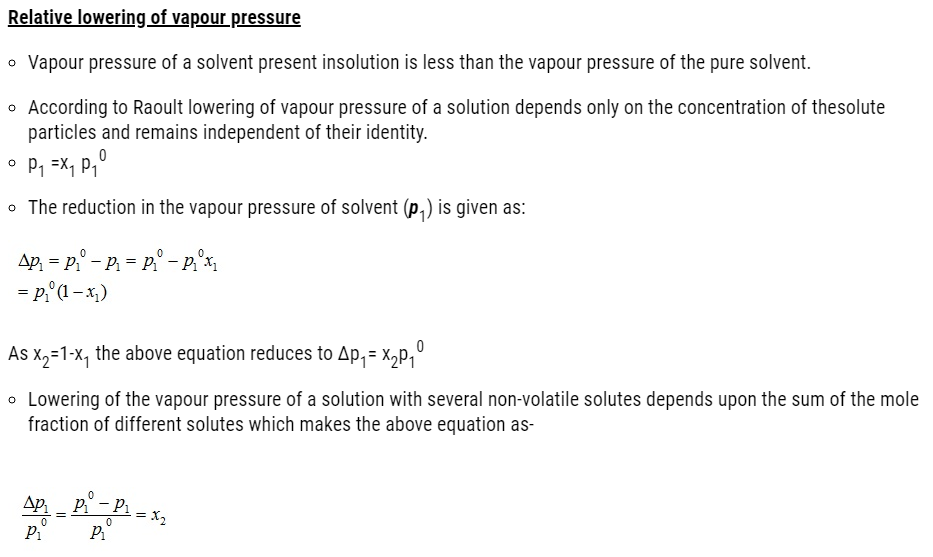
- Vapour pressure of solution decreases on addition of a non-volatile solute to a volatile solvent.
- Properties of solutions affected by the decrease of vapour pressure includes-
- Relative lowering of vapour pressure of the solvent
- Depression of freezing point of the solvent
- Elevation of boiling point of the solvent and
- Osmotic pressure of the solution.
- These properties of solution depend on the number of solute particles present in the solution regardless of their nature relative to the total number of particles present in the solution. These properties are termed as colligative properties derived from a Latin word with co meaning together ligare meaning to bind.

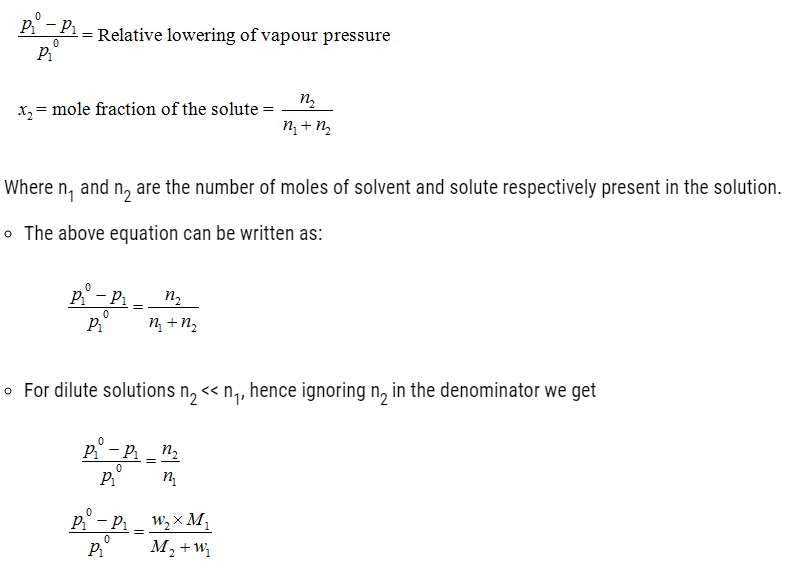
w1 and M1 = Masses and molar masses of solvent.
w2 and M2 = Masses and molar masses of solute.
Elevation of boiling point
- Increase in temperature increases the vapour pressure of a
- A liquid boils at atemperature where the vapour pressure is equal to the atmospheric
- Vapourpressure of the solvent decreases in the presence of non-volatile solute.
- The boilingpoint of a solution is always higher than that ofthe boiling point of the pure solvent in which thesolution is prepared as.
- The elevation ofboiling point also depends on the number ofsolute molecules rather than their nature.
- The increase in the boiling point = ΔTb = Tb – Tb0. This is known as elevation of boiling point.
Tb0 = boiling point of pure solvent
Tb0 = boiling point of solution
- In dilute solutions the elevation of boiling point Tb is directly proportional to the molal concentration of the solute in a solution. Mathematically,
ΔTb ∝ m
ΔTb = kb m
m = Molality = number of moles of solute dissolved in 1 kg of solvent
Kb = constant of proportionality. It is also known as Boiling Point Elevation Constant or Molal Elevation Constant (Ebullioscopic Constant). The unit of Kb is K kg mol-1.
- Let w2 and M2 = Masses and molar masses of solute
w1= Mass of solvent
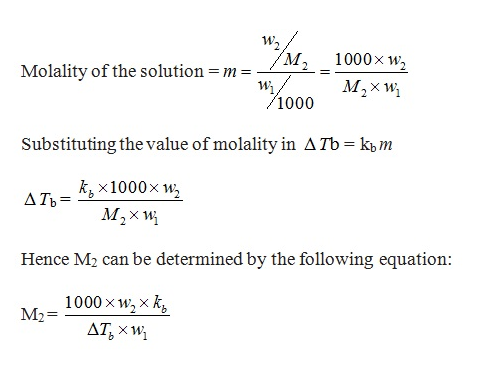
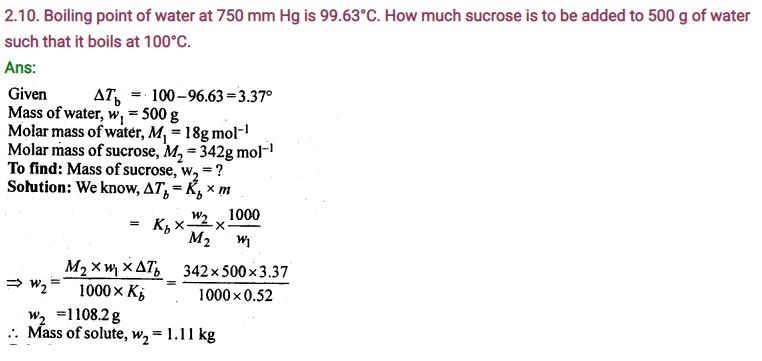
Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose is to be added to 500 g of water such that it boils at 100°C.Molal elevation constant for water is 0.52 K kg mol-1.
Sol. Elevation of boiling point ΔTb= (100 + 273) – (99.63 + 273) = 0.37 K
Mass of water, wl = 500 g
Molar mass of sucrose (C12H22O11),
M2= 11 × 12 + 22 × 1 + 11 × 16 = 342 g mol – 1
Molal elevation constant, Kb= 0.52 K kg mol – 1
We know that: