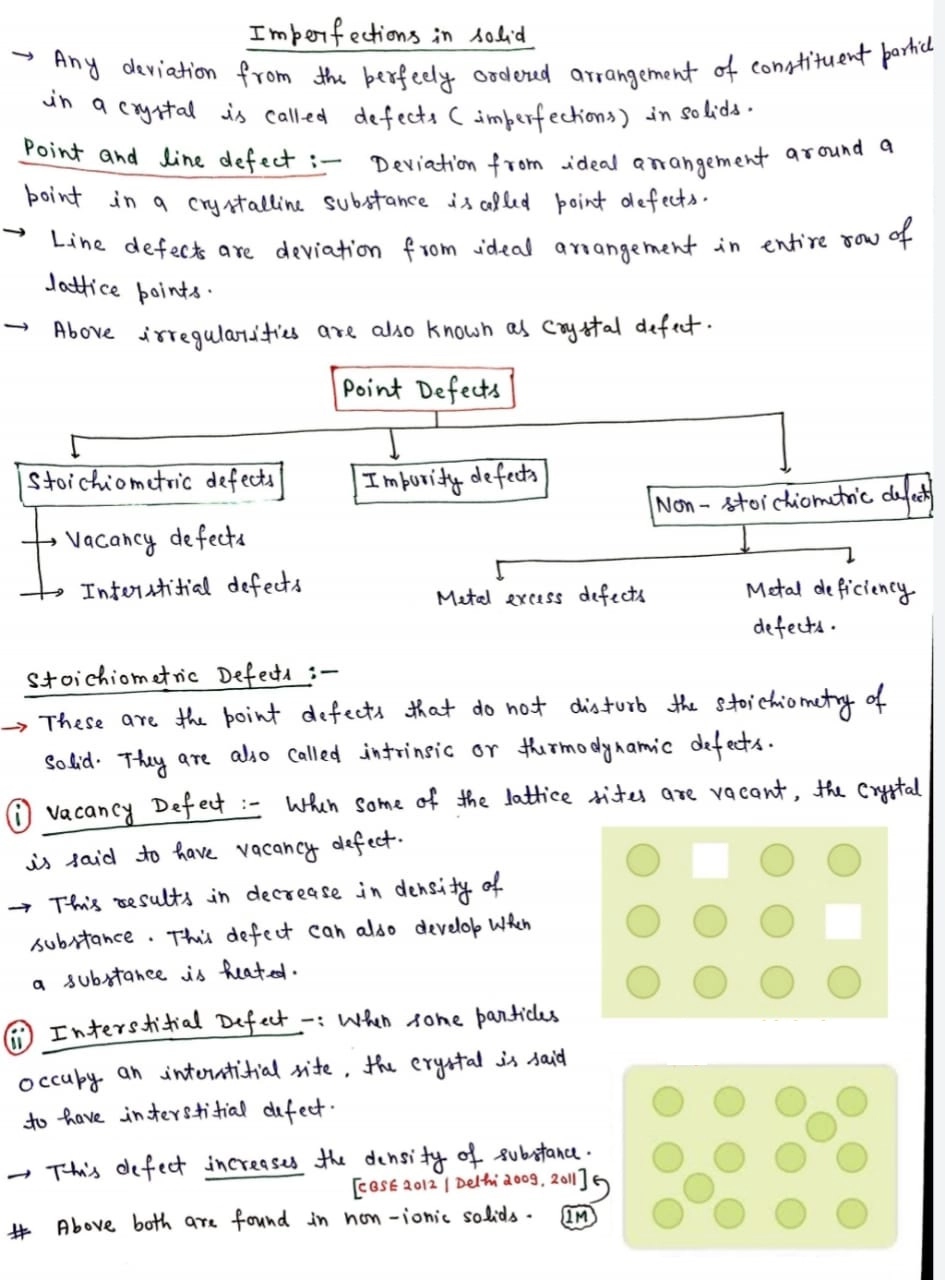
Imperfections in Solids
- Constituent particles in crystalline solids are arranged in a short range as well as long range
order yet crystals are not perfect. - They possess small crystals have defects that are irregularities in the arrangement of constituent
particles in them when crystallization process occurs at fast or moderate rate. - These defects are of two types- point defects and line defects.
- The irregularities or deviations from ideal arrangement around a point or an atom in a
crystalline substance. - The irregularities or deviations from ideal arrangement in entire rows of lattice points are known as line defects.
- These irregularities are called crystal defects.
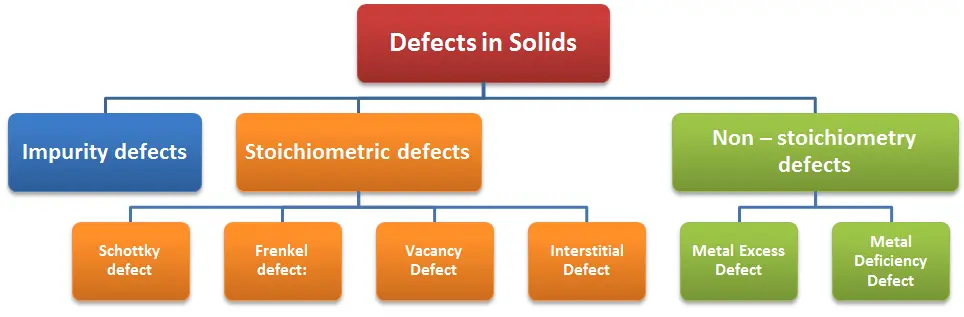
- Point defects can be classified into three types: (i) stoichiometric defects
(ii) impurity defects and (iii) non-stoichiometric defects


Q. Which type of defect increase the density of substance & which type of solid found this defect? [CBSE 2012/Delhi 2009, 2011]
Ans. Interstitial Defect:- When some particles occupy an interstitial site, the crystal is to have interstitial defect. This defect increase the density of substance.
Vacancy & Interstitial defect both found in non-ionic solids.
Q. In which defect large size difference between cations and anions founds? [CBSE 2015C/2012/2010C/ Delhi 2013]
Ans. Frenkel Defect
Q. Which compound shows both Frenkel and Schottky defect?[Delhi 2014/2015]
Ans. AgBr shows both frenkel and schottky defect.
Q. In which defect density decrease? [Delhi 2010/CBSE 2009]
Ans. Density decrease in schottky defect/vacancy defect.
Q. In which defect density does not change? [Delhi 2009/2010]
Ans. Density does not change in Frenkel Defect.
Q. In which defect density increase? [Delhi 2009/2011, CBSE 2012]
Ans. Density increases in Interstitial defect.
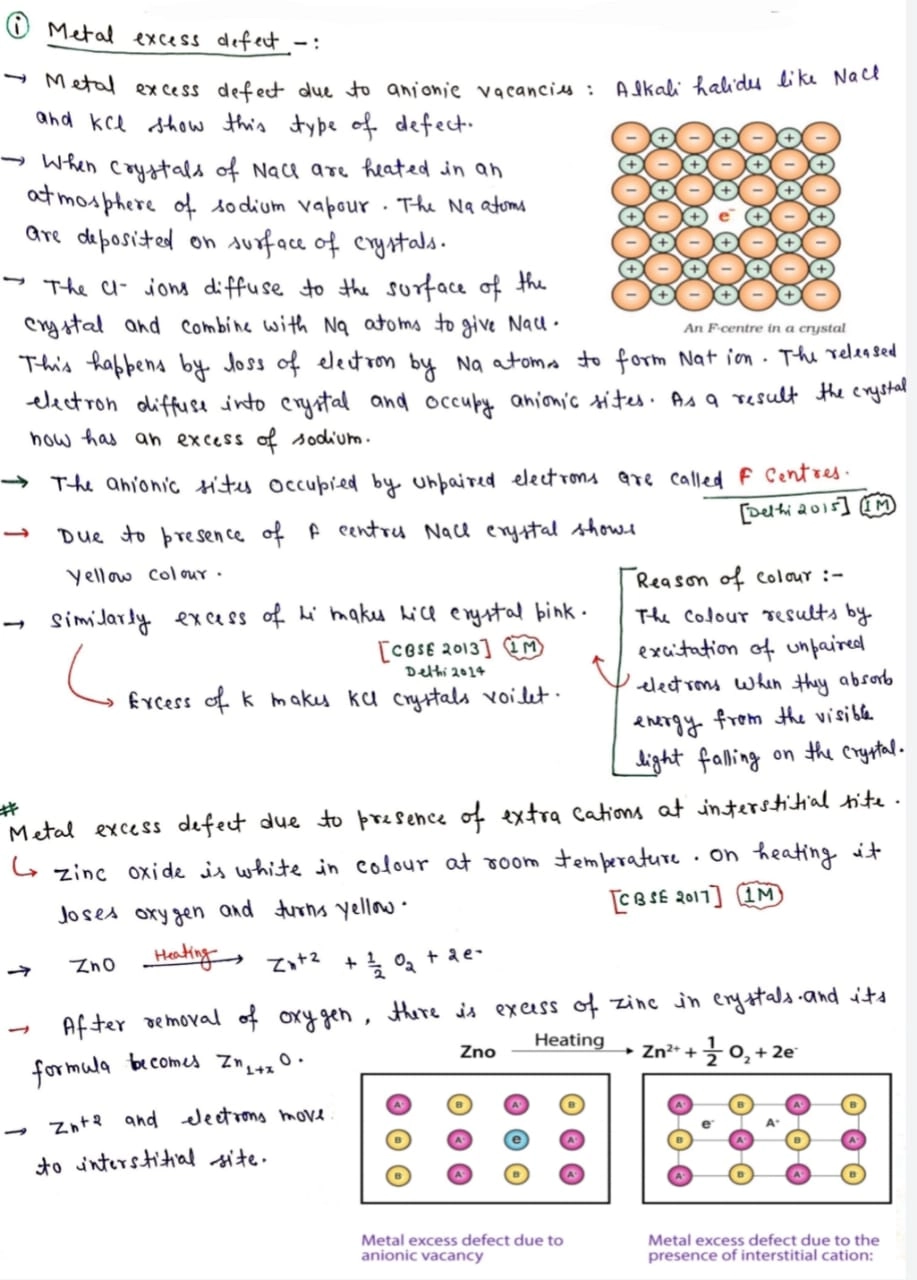
Q. Define F Centres ? [Delhi 2015]
Ans. In metal excess defect, The anionic sites occupied by unpaired electrons are called F Centres.
Due to presence of F centres NaCl crystal shows yellow colour.
Q. Excess of Li makes LiCl crystal pink (Why) & Excess of K makes KCl crystal violet (Why) [CBSE 2013/Delhi 2014/Delhi 2015]
Ans. Reason of colour:- The colour results by excitation of unpaired electrons when they absorb energy from the visible light falling on the crystal.
NCERT Question
1.19. What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
Ans: When a solid is heated, vacancy defect is produced in the crystal. On heating, some atoms or ions leave the lattice site completely, i.e., lattice sites become vacant. As a result-of this defect, density of the substances decreases.
1.20. What types of stoichiometric defects are shown by (C.B.S.E. Delhi 2013)
(i) ZnS
(ii) AgBr?
Ans:
(i) ZnS crystals may show Frenkel defects since the cationic size is smaller as compared to anionic size.
(ii) AgBr crystals may show both Frenkel and Schottky defects.
1.21. Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
Ans: Let us take an example NaCl doped with SrCl, impurity when SrCl2 is added to NaCl solid as an impurity, two Na+ ions will be replaced and one of their sites will be occupied by Sr21- while the other will remain vacant. Thus, we can say that when a cation of higher valence is added as an impurity to an ionic solid, two or more cations of lower valency are replaced by a cation of higher valency to maintain electrical neutrality. Hence, some cationic vacancies are created.
1.22. Ionic solids, which have anionic vacancies due to metal excess defect, develop colour. Explain with the help of a suitable example.
Ans: Let us take an example of NaCl. When NaCl crystal is heated in presence of Na vapour, some Cl–ions leave their lattice sites to combine with Na to form NaCl. The e-1 s lost by Na to form Na+ (Na+ + Cl–—> NaCl) then diffuse into the crystal to occupy the anion vacancies. These sites are called F-centres. These e-s absorb energy from visible light, get excited to higher energy level and when they fall back to ground state, they impart yellow colour to NaCl crystal.

(ii) Frenkel defect : This defect arises when some of the ions in the lattice occupy interstitial sites leaving lattice sites vacant. This defect is generally found in ionic crystals where anion is much larger in size than the cation, e.g., AgBr, ZnS, etc. Due to this defect density does not change, electrical conductivity increases to a small extent and there is no change in overall chemical composition of the crystal.
(iii) Interstitial defect : When some constituent particles (atoms or molecules) occupy an interstitial site of the crystal, it is said to have interstitial defect. Due to this defect the density of the substance increases.
(iv) F-Centres : These are the anionic sites occupied by unpaired electrons. F-centres impart colour to crystals. The colour results by the excitation of electrons when they absorb energy from the visible light falling on the crystal.
Question 25. ( The Solid State NCERT Solution )
If NaCI is doped with 10-3 mol % SrCl2, what is the concentration of cation vacancies?
Solution:
Let moles of NaCI = 100
∴ Moles of SrCl2 doped = 10-3
Each Sr2+ will replace two Na+ ions. To maintain electrical neutrality it occupies one position and thus creates one cation vacancy.
∴ Moles of cation vacancy in 100 moles NaCI = 10-3
Moles of cation vacancy in one mole
NaCI = 10-3 × 10-2 = 10-5
∴ Number of cation vacancies
= 10-5 × 6.022 × 1023 = 6.022 × 1018 mol-1