Electrical resistance
- It is denoted by the symbol ‘R’.
- It is measured in a unit called ohm (Ω) with the help of a Wheatstone bridge
- The electrical resistance is:
- Directly proportional to its length, l
- Inversely proportional to its area of cross section, A.

Electrical conductance
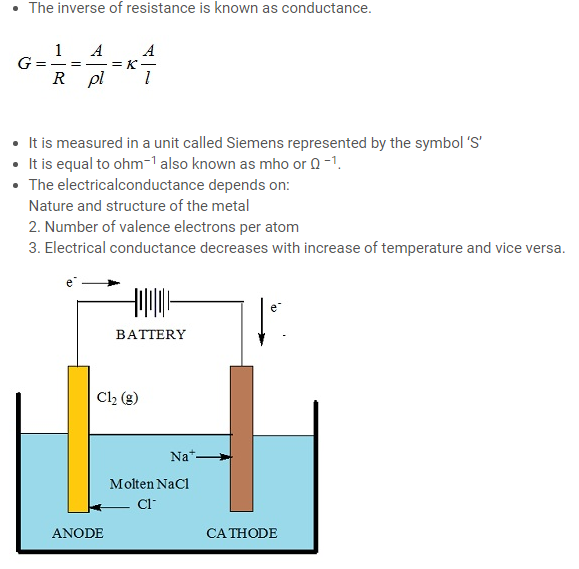
- The free ions Na+ and Cl– present in the solution are responsible for the conductance in a solution.
- The inverse of resistivity is termed as conductivity.
- Ω = 1/k
- It is represented by the symbol (Greek, kappa).
- It is measured in a unit called Sm–1.
- When length = 1m.
Cross sectional area = 1m2
Then conductivity becomes the conductance.
The conductivity of an electrolytic solution depends on:
1.Nature of the electrolyte added
2.Size of the ions produced and their solvation
3.Nature of the solvent and its viscosity
4.Concentration of the electrolyte
5.It increases with the increase of temperature.
6.Pressure
- Matters can be classified into conductors, insulators andsemiconductors depending on the magnitude of their conductivity.
Conductors:
- Solids with conductivities ranging between 104 to 107 ohm–1m–1 are conductors.
- Metals have conductivities in the order of 107 ohm–1m–1 is good conductors.
- For example, Iron, Copper, Aluminum.

Fig. Wood and plastics are also solids but are insulators
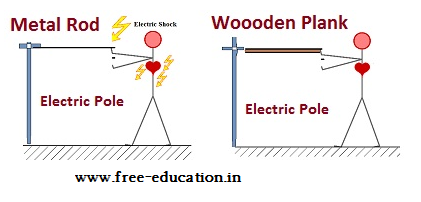
A man touching the electric pole with a metal rod will get an electric shock because metal rod is a conductor whereas a man touching the same with a wooden plank will be safe because wood is an insulator.
Semiconductors:
- Solids with conductivities in the intermediate range from 10–6 to 104 ohm–1 m–1.
- For example, Gallium, Germanium, Silicon
Measurement of resistance of a solution of an electrolyte

PROBLEM:
THE RESISTANCE OF A CONDUCTIVITY CELL CONTAINING 0.001M KCL SOLUTION AT 298 K IS 1500 Ω. WHAT IS THE CELL CONSTANT IF CONDUCTIVITY OF 0.001M KCL SOLUTION AT 298 K IS 0.146 X 10-3 S CM-1.
SOLUTION:
Conductivity, k = 0.146 × 10 – 3 S cm – 1
Resistance, R = 1500 Ω
Cell constant = k × R = 0.146 × 10 – 3 × 1500 = 0.219 cm – 1