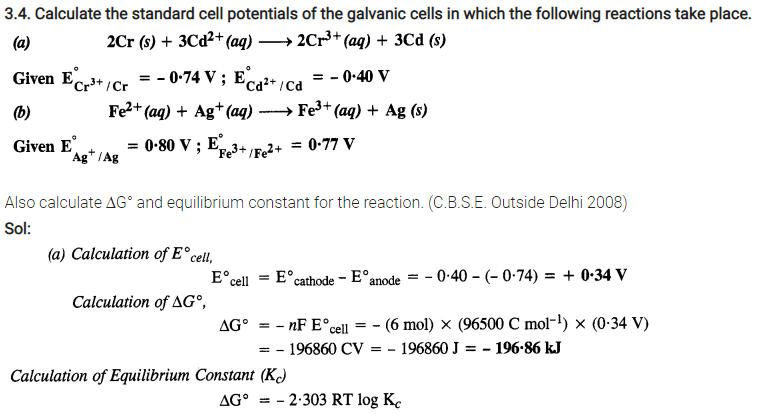
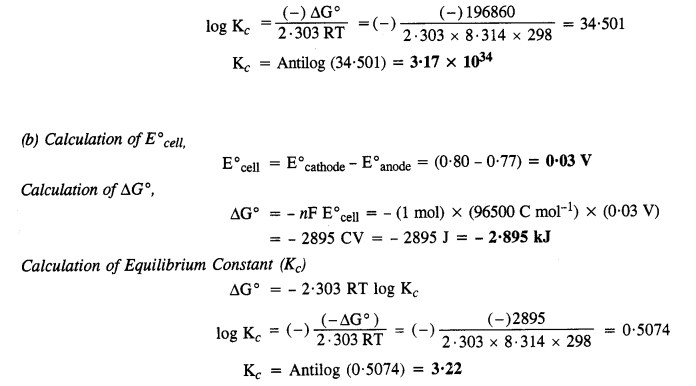
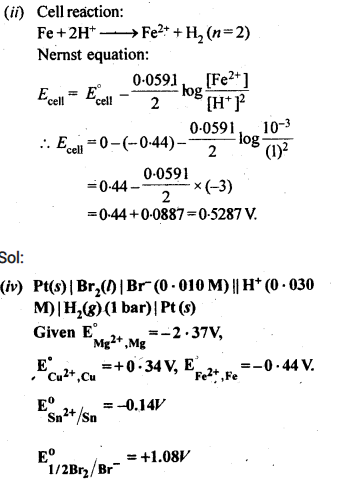
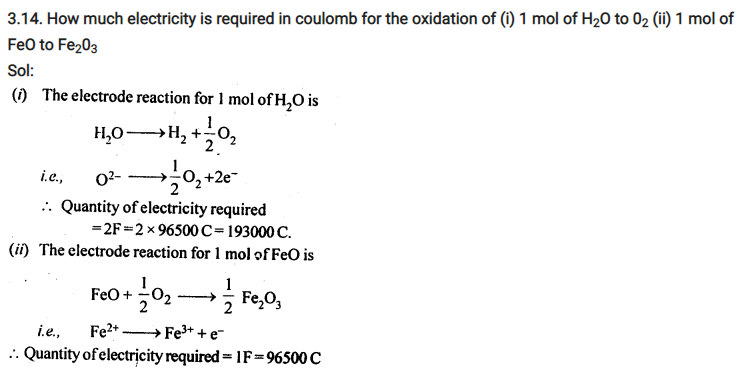
NCERT Solutions For Class 12 Chemistry Chapter 3 Electrochemistry




NCERT EXERCISES
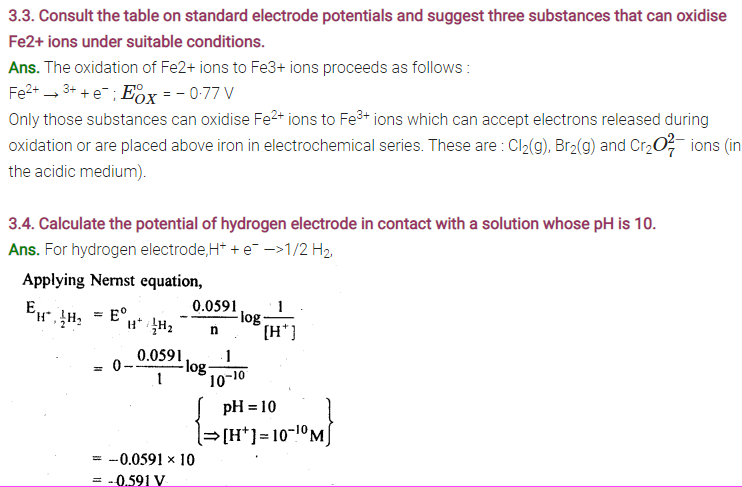
3.1. Arrange the following metals in the order in which they displace each other from their salts.
Al, Cu, Fe, Mg and Zn
Sol: Mg, Al, Zn, Fe, Cu.
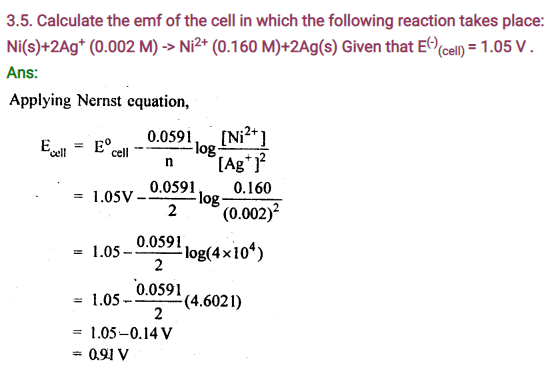
3.2. Given the standard electrode potentials, K+/K=-2. 93 V, Ag+/Ag = 0.80 V, Hg2+/Hg =0.79V, Mg2+/Mg=-2.37V, Cr3+/Cr=0.74V.
Arrange these metals in their increasing order of reducing power.
Sol: Higher the oxidation potential more easily it is oxidized and hence greater is the reducing power. Thus, increasing order of reducing power will be Ag<Hg<Cr<Mg<K.
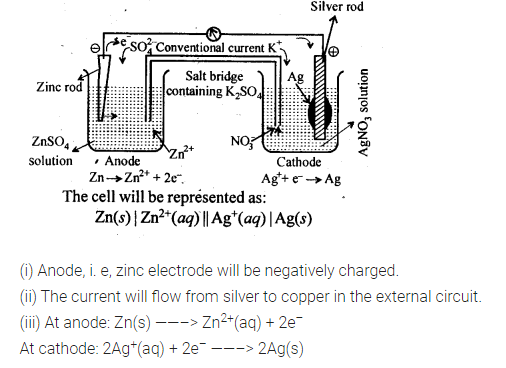
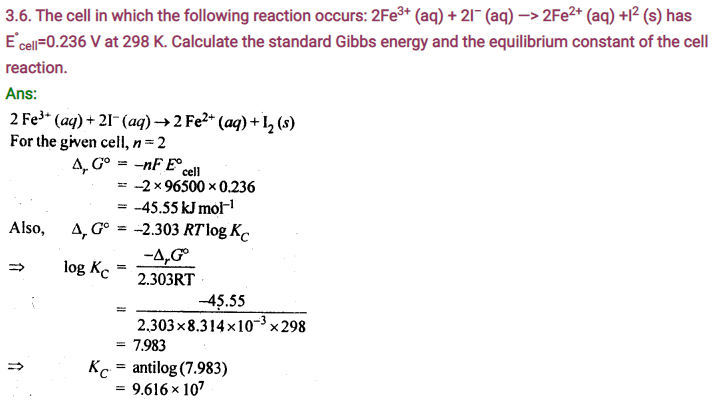
3.3. Depict the galvanic cell in which the reaction
Zn(s) + 2Ag+(aq) —-> 7M2+(aq) + 2Ag (s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Sol. The set-up will be similar to as shown below,