Class 12th Chemistry Chapter 4 Chemical Kinetic
Introduction
The stream of chemistry that governs the rate of reactions along with their mechanisms is termed as Chemical kinetics derived from a Greek word meaning chemical movement.
Combination of two or more reactants to produce a new product is called reaction.
Elementary Reaction:
The reaction that occurs in a single step to give the product is called an elementary reactions.
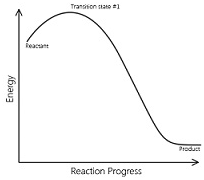
Complex reaction:
The reactions that occur as a result of sequence of elementary reactions to give the product is called complex reactions.
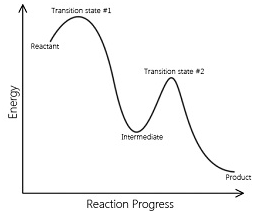
Rate of reaction
The rate at which the concentration of reactant or product participating in a chemical reaction alters is called rate of reaction.
Rate of reaction = change in concentration/ time = (mol/litre)/time
Reactant (R) –> Product.
Rate [R]
Rate = k[R]
k = rate constant or velocity constant.
Let one mole of the reactant A produce one mole of the product B.
Let at time t1
[A]1 and [B]1 = Concentrations of A and B
Let at time t2
[R]2 and [P]2 = Concentrations of A and B
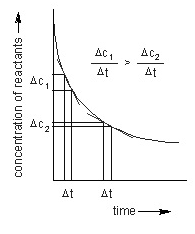
Rate of disappearance of A = Decrease in concentration of R / Time taken = -∆[A]/∆t
Rate of appearance of B = Increase in concentration of P / Time taken = +∆[B]/∆t
When two or more reactants combine with each other the molecules of the respective reactants collide with each other to form the product. The collision between the molecules increases with the increase in concentration of the reactants and thereby increases the rate of reaction. A + B –> C + D
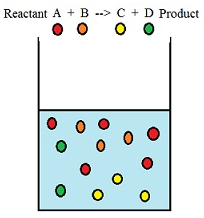
Here molecules of reactant A and B collide to produce molecules of product C and D.
Therefore we can conclude that rate of reaction is directly proportional to the concentration of the participating reactants.
Rate ∝ [A]x [B]y
Or Rate = k[A]x [B]y
Hg(l) + Cl2 (g) –> HgCl2(s)
Rate of reaction= -∆[Hg]/∆t = ∆[Cl2]/∆t = ∆[Hg Cl2]/∆t
Factors affecting rate of reaction
Nature of reactant
Nature of bonding in the reactants determines the rate of a reaction. The ionic compounds react faster compared to covalent compounds due to requirement of energy in covalent compounds to cleave the existing binds.
The reaction between ionic compounds:
Precipitation of AgCl
AgNO3 + NaCl –> AgCl + NaNO3
The reactions between covalent compounds:
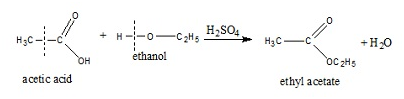
Temperature
Rate of reaction increases with the rise in temperature due to increase in average kinetic energy which in turn increases the number of molecules having greater energy than threshold energy and consequently increasing the number of effective collisions. The rate of a reaction is doubled (i.e., increased by 100%) with 10 oC rise in temperature.
Pressure
Increase in partial pressure increases the number of collisions. Therefore, the rate of reactions involving gaseous reactants increases with the increase in partial pressures.
Catalyst
A catalyst increases the rate of reaction by giving an alternative path with lower activation energy (Ea’) for the reaction to proceed.
Concentration of reactants
Increase in concentration increases the number of collisions and the activated collisions between the reactant molecules. According to the collision theory, rate is directly proportional to the collision frequency. Consequently, the rate of a reaction increases with the rise in the concentration of reactant.
Surface area
The rate of a reaction increases with increase in the surface area of solid reactant.
PROBLEM. For the reaction: 2A + B → A2B , The rate = k[A][B]2with k= 2.0 x 10-6mol-2L2s-1. Calculate the initial rate of the reaction when [A] = 0.1 mol L-1, [B] = 0.2 mol L-1. Calculate the rate of reaction after [A] is reduced to 0.06 mol L-1.
SOLUTION. Rate = k [A][B]2
= (2.0 × 10 – 6mol – 2L2s – 1) (0.1 mol L – 1) (0.2 mol L – 1)2
= 8.0 × 10 – 9mol – 2L2s – 1
Reduction of [A] from 0.1 mol L – 1to 0.06 mol – 1
The concentration of A reacted = (0.1 – 0.06) mol L – 1 = 0.04 mol L – 1
The concentration of B reacted= 1/2 x 0.04 mol L-1 = 0.02 mol L – 1
The concentration of B available, [B] = (0.2 – 0.02) mol L – 1
= 0.18 mol L – 1
After reduction of [A] to 0.06 mol L – 1
The rate of the reaction
Rate = k [A][B]2
= (2.0 × 10 – 6mol – 2L2s – 1) (0.06 mol L – 1) (0.18 mol L – 1)2
= 3.89 mol L – 1s – 1