Let’s Understand what are Characteristics of solid-state
General Characteristics of Solid State
- Definite shape due to strong intermolecular forces of attraction.
- They have distinct boundaries.
- Fixed volume.
- They cannot flow.
- Solid have negligible compressibility due to negligible distance between the neighbouring molecules.
- Solid possess a tendency to uphold their shape when exposed to external force.
- They break under force but it is difficult to change their shape so they are rigid.
- They have high density and do not diffuse at all.
The State of Solid: Solid is Classified into two categories:-
- Crystalline Solid
- Amorphous Solid
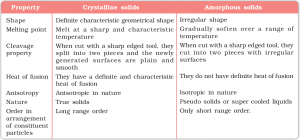
Crystalline Solid:-
- This range of solids consists of a broad range of small crystals having a definite characteristic geometrical shape.
- The constituent particles are arranged in a long range order (symmetry and regularity of arrangement of constituent particles that repeat at any distance from a given atom due to the interaction between the particles) with a regular and periodically repeating pattern over the entire crystal.
- Crystalline solids possess a sharp melting point.
- Crystalline solids are anisotropic in nature due to different arrangement of particles in different directions. This leads to different value of physical property along different directions in the same crystals.
- Metallic elements including iron, copper and silver are typical examples of crystalline solids.
- On the other hand non – metallic elements like sulphur, phosphorus and iodine and compounds like sodium chloride, zinc sulphide and naphthalene and quartz are typical examples of crystalline solids.

Amorphous Solid :-
- The term amorphous solid is derived form a Greek word amorphous meaning no form.
- The constituent particles are arranged in a short range order with a regular and periodically repeating pattern over short distances.
- These solids get softened at a certain temperature and hence can be moulded and drawn into various desired shapes. Solids may also acquire crystalline form at some temperature when heated.
- These solids have the ability to flow very slowly due to which they are also termed as pseudo solids or super cooled liquids.
- These solids are isotropic in nature due to the absence of long range order and irregular arrangement of the constituent particles in all direction. This leads to same value of physical property along all the direction.
- Irregular arrangement of particles in an amorphous solidGlass, rubber, amorphous silicon and plastics are typical examples of amorphous solids.
- Amorphous silicon is a photovoltaic material widely used for conversion of sunlight into electricity.
Difference between crystalline and Amorphous Solid
Anisotropic Properties and Isotropic Properties
:- Anisotropic Properties:- It can be defined as a difference in material’s physical or mechanical properties (like refractive index, conductivity, tensile strength) in different direction.
Crystalline solids are anisotropic in nature. This anisotropy in crystals is due to different arrangement of particles along different direction.

Isentropic :- Amorphous solids are isotropic in nature. It is because there is no long range order in them and arrangement is irregular along all the direction so value of any physical properties would be same along any direction.
Q. Why are solids rigid? (NCERT Book)
Ans: The constituent particles in solids have fixed positions and can oscillate about their mean positions. Hence, they are rigid.
Q. Why do solids have definite volume?(NCERT Book)
Ans: Solids keep their volume because of rigidity in their structure. The interparticle forces are very strong. Moreover, the interparticle spaces are very few and small as well. As a result, their volumes cannot change by applying pressure.
Q. Why is glass considered as super cooled liquid ? (C.B.S.E. Delhi 2013) (NCERT Book)
Ans: Glass is considered to be super cooled liquid because it shows some of the characteristics of liquids, though it is an amorphous solid. For example, it is slightly thicker at the bottom. This can be possible only if it has flown like liquid, though very slowly.
Q. Define the term ‘amorphous’. Give a few examples of amorphous solids.(NCERT Book)
Sol. Amorphous solids are those substances, in which there is no regular arrangement of its constituent particles, (i.e., ions, atoms or molecules). The arrangement of the constituting particles has only short-range order, i.e., a regular and periodically repeating pattern is observed over short distances only, e.g., glass, rubber, and plastics.
Q. What makes glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?(NCERT Book)
Sol. Glass is a supercooled liquid and an amorphous substance. Quartz is the crystalline form of silica (SiO2) in which tetrahedral units SiO4 are linked with each other in such a way that the oxygen atom of one tetrahedron is shared with another Si atom. Quartz can be converted into glass by melting it and cooling the melt very rapidly. In the glass, SiO4 tetrahedra are joined in a random manner.
Q. Why crystalline solids are anisotropic in nature? (CBSE 2014C/Delhi 2011)
Ans. Crystalline solid are anisotropic in nature. This anisotropy in crystals is due to different arrangement of particles along different direction.
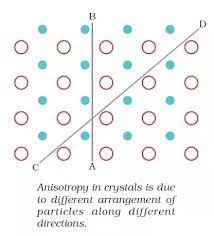
A —-> B : Regular Pattern, C —-> D : Regular Pattern
But in A – B is different form C – D
Q. Difference between Crystalline and Amorphous Solid? (CBSE 2008C/ Delhi 2017)
Ans. For Answer see above.
Q. Classify the following as amorphous or crystalline solids: Polyurethane, naphthalene, benzoic acid, Teflon, potassium nitrate, cellophane, polyvinyl chloride, fibreglass, copper (NCERT Books)
Ans: Crystalline solids: Benzoic acid, potassium nitrate, copper Amorphous solids: Polyurethane, Teflon, cellophane, polyvinyl chloride, fibreglass
Q. Refractive index of a solid is observed to have the same value along all directions. Comment on the nature of this solid. Would it show cleavage property?
Ans: As the solid has same value of refractive index along all directions, it is isotropic in nature and hence amorphous. Being amorphous solid, it will not show a clean cleavage and when cut, it will break into pieces with irregular surfaces.