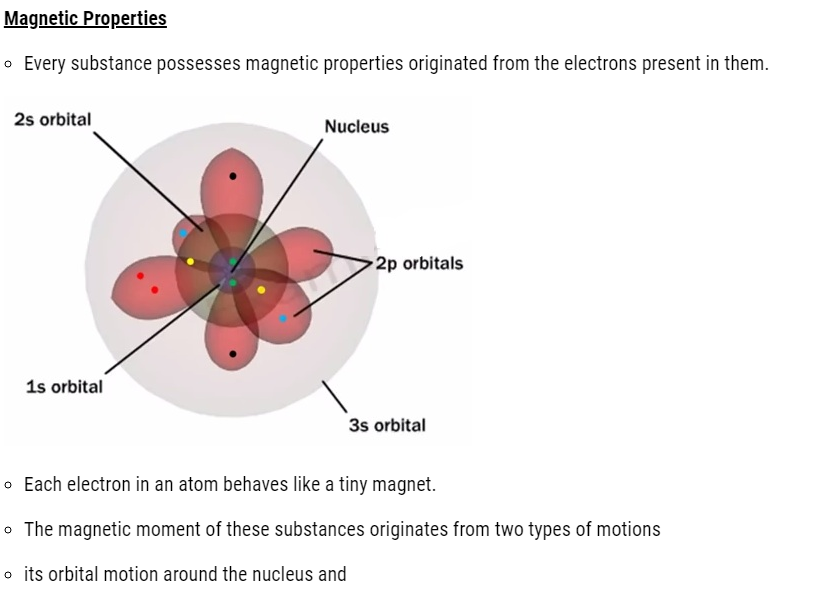
- its spin around its own axis.
- Electron being a charged particle undergoes these motions and can be considered as a small loop of current possessing a magnetic moment.
- Therefore, each electron has a permanent spin and an orbital magnetic moment associated with it.
- Magnitude of this magnetic moment is very small and is measured in the unit called Bohr magneton, μ B and is equal to 9.27 × 10–24A m2.
On the basis of their magnetic properties, substances can be classified into five categories:
- Paramagnetic
- Diamagnetic
- Ferromagnetic
- Antiferromagnetic
- Ferrimagnetic.


Ferromagnetism
- Ferromagnetic substances get strongly attracted towards magnetic field.
- They can be permanently magnetized.
- In solid state, the metal ions of ferromagnetic substances are grouped together into small regions and are known as domains that act as a tiny magnet.
- In an unmagnetised ferromagnetic substance the domains are randomly oriented that cancels out their magnetic moments.
- When placed in a magnetic field all the domains of the substance get oriented in the direction of the magnetic field producing a strong magnetic effect which persists even on removal of the magnetic field and the ferromagnetic substance becomes a permanent magnet.
- For example, iron, cobalt, nickel, gadolinium and CrO2 are ferromagnetic substances.


1.24. What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic. Justify your answer. (NCERT Book)
Ans: Ferromagnetic substances make better permanent magnets. This is because when placed in magnetic field, their domains get oriented in the directions of magnetic field and a strong magnetic field is produced. This ordering of domains persists even when external magnetic field is removed. Hence, the ferromagnetic substance becomes a permanent magnet.
Question 26. ( The Solid State NCERT Solution )
Explain the following with suitable example:
- Ferromagnetism
- Paramagnetism
- Ferrimagnetism
- Antiferromagnetism
- 12-16 and 13-15 group compounds.
Solution:
(i) Ferromagnetic substances : Substances which are attracted very strongly by a magnetic field are called ferromagnetic substances, e.g., Fe, Ni, Co and CrO2 show ferromagnetism. Such substances remain permanently magnetised, once they have been magnetised. This type of magnetic moments are due to unpaired electrons in the same direction.The ferromagnetic material, CrO2, is used to make magnetic tapes used for audio recording.
(ii) Paramagnetic substances : Substances which are weakly attracted by the external magnetic field are called paramagnetic substances. The property thus exhibited is called paramagnetism. They are magnetised in the same direction as that of the applied field. This property is shown by those substances whose atoms, ions or molecules contain unpaired electrons, e.g., O2, Cu2+, Fe3+, etc. These substances, however, lose their magnetism in the absence of the magnetic field.
(iii) Ferrimagnetic substances : Substances which are expected to possess large magnetism on the basis of the unpaired electrons but actually have small net magnetic moment are called ferrimagnetic substances, e.g., Fe3O4, ferrites of the formula M2+Fe2O4 where M = Mg, Cu, Zn, etc. Ferrimagnetism arises due to the unequal number of magnetic moments in opposite direction resulting in some net magnetic moment.
(iv) Antiferromagnetic substances : Substances which are expected to possess paramagnetism or ferromagnetism on the basis of unpaired electrons but actually they possess zero net magnetic moment are called antiferromagnetic substances, e.g., MnO. Antiferromagnetism is due to the presence of equal number of magnetic moments in the opposite directions
(v) 13-15 group compounds : When the solid state materials are produced by combination of elements of groups 13 and 15, the compounds thus obtained are called 13-15 compounds. For example, InSb, AlP, GaAs, etc.
12-16 group compounds : Combination of elements of groups 12 and 16 yield some solid compounds which are referred to as 12-16 compounds. For example, ZnS, CdS, CdSe, HgTe, etc. In these compounds, the bonds have ionic character.