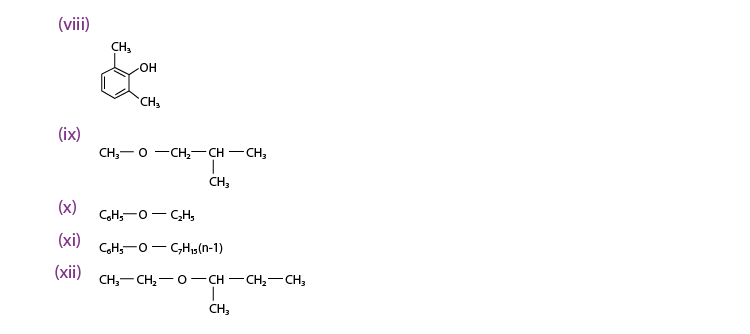
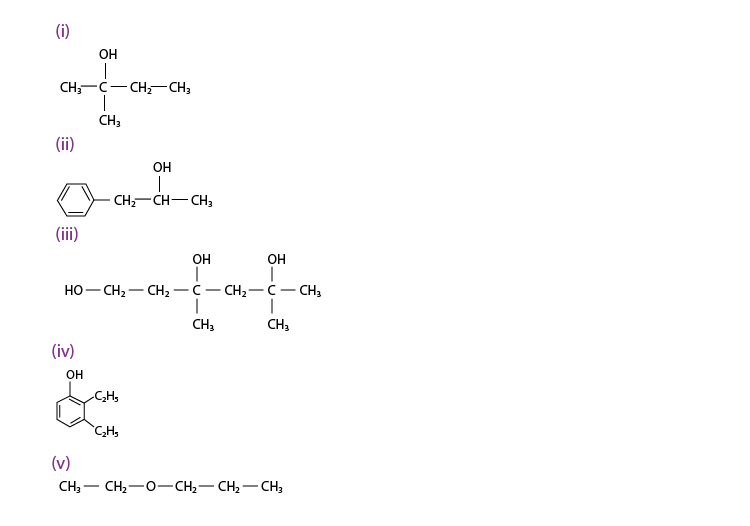
Q 11.1 :
Write IUPAC names of the following compounds:
Solution :
(i) 2, 2, 4 -Trimethylpentan – 3 – ol
(ii) 5 – Ethylheptane – 2, 4 – diol
(iii) Butane – 2, 3 – diol
(iv) Propane – 1, 2, 3 – triol
(v) 2-Methylphenol
(vi) 4 – Methyl phenol
(vii) 2, 5 – Dimethylphenol
(viii) 2, 6 – Dimethylphenol
(ix) 1 – Methoxy – 2 – methyl propane
(x) Ethoxy benzene
(xi) 1 – Phenoxyheptane
(xii) 2 – Ethoxybutane
Q 11.2 :
Write structures of the compounds whose IUPAC names are as follows:
(i) 2 – Methylbutan – 2 – ol
(ii) 1 – Phenylpropan – 2 – ol
(iii) 3 , 5 – Dimethylhexane – 1 , 3 , 5 – triol
(iv) 2, 3 – Diethylphenol
(v) 1 – Ethoxypropane
(vi) 2 – Ethoxy – 3 – methylpentane
(vii) Cyclohexylmethanol
(viii) 3 – Cyclohexylpentan – 3 – ol
(ix) Cyclopent – 3 – en – 1 – ol
(x) 4-Chloro-3-ethylbutan-1-ol.
Solution :
Q 11.3 :
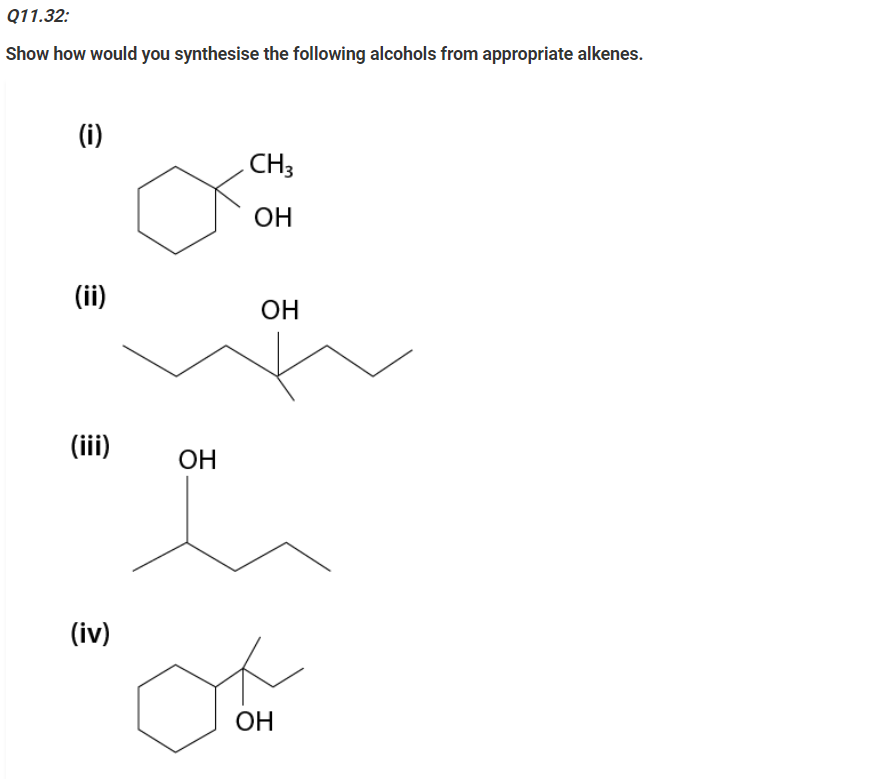
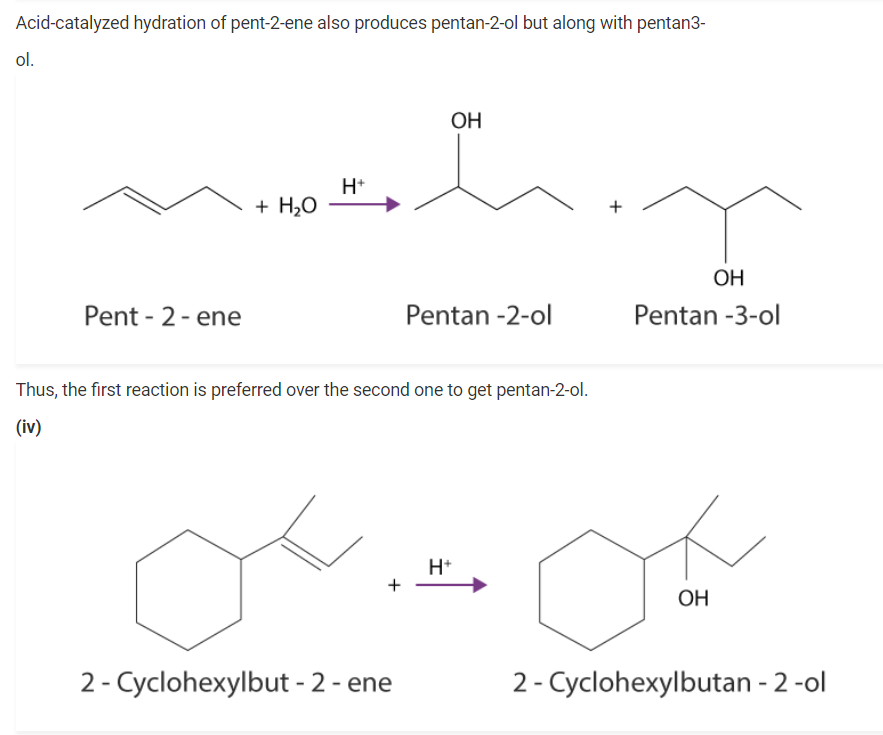
(i) Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
(ii) Classify the isomers of alcohols in question.
Solution :
(i) The structures & IUPAC names of all isomeric alcohols with a molecular formula of C5H12O are shown below:
(a) CH3-CH2-CH2-CH2-CH2-OH
Pentan – 1 – ol (1 °)
2 – Methylbutan – 2 – ol ( 3 ° )
(ii) Primary alcohol: Pentan – 1 – ol ; 2 – Methylbutan – 1 – ol ;
3 – Methylbutan – 1 – ol ; 2, 2 – Dimethylpropan – 1 – ol
Secondary alcohol: Pentan – 2 – ol ; 3 – Methylbutan – 2 – ol ;
Pentan – 3 – ol
Tertiary alcohol: 2 – methylbutan – 2 – ol
Q 11.4:
Explain why propanol has higher boiling point than that of the hydrocarbon, butane.
Solution:
The presence of – OH group makes Propanol undergo intermolecular H-bonding. Butane, while on the other side does not have the same privilege
Hence, additional energy would be required to break the intermolecular hydrogen bonds.
This is the reason why hydrocarbon butane has a lower boiling point than propanol.
Q 11.5:
Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Solution :
Due to the presence of – OH group, alcohols form H – bonds with water.
As a result, alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses.
Q 11.6:
What is meant by the hydroboration-oxidation reaction? Illustrate it with an example.
Solution :
The hydroboration – oxidation reaction is the reaction where borane is added in order for the oxidation to take place. For example, propan – 1 – ol is formed by making propene undergo the hydroboration – oxidation reaction. In the above reaction, the reaction between propene and diborane ( BH 3 )2 takes place in order to form trialkyl borane which acts an additional product. This additional product is oxidized to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide.
Q 11.7:
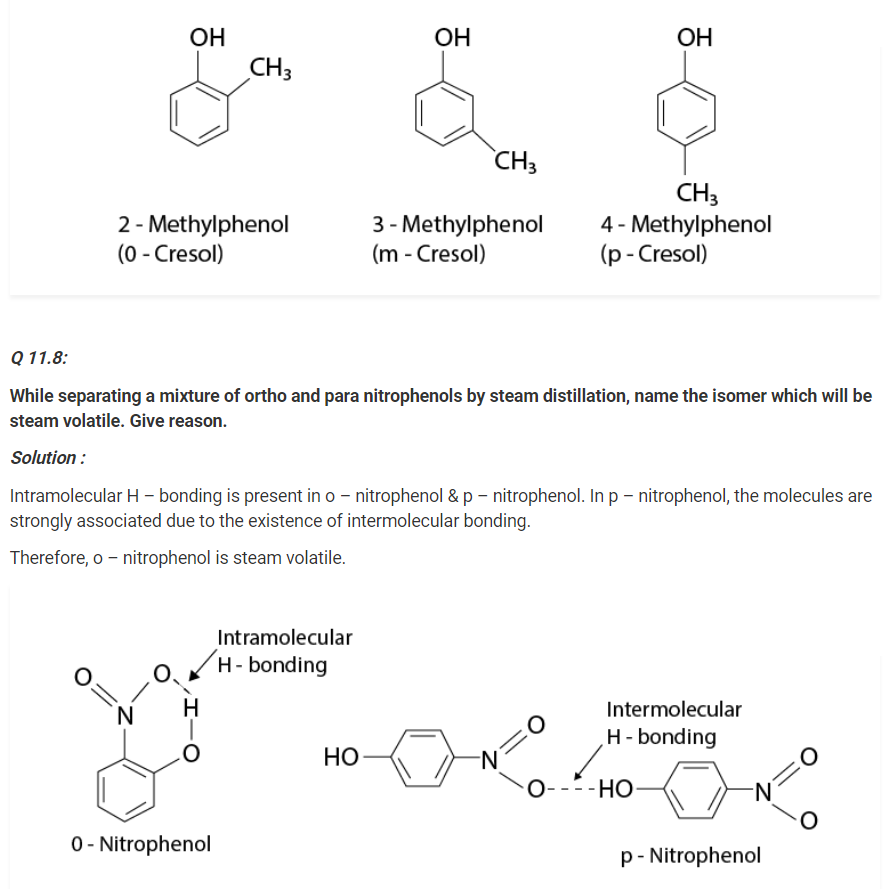
Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
Solution :
Q 11.10:
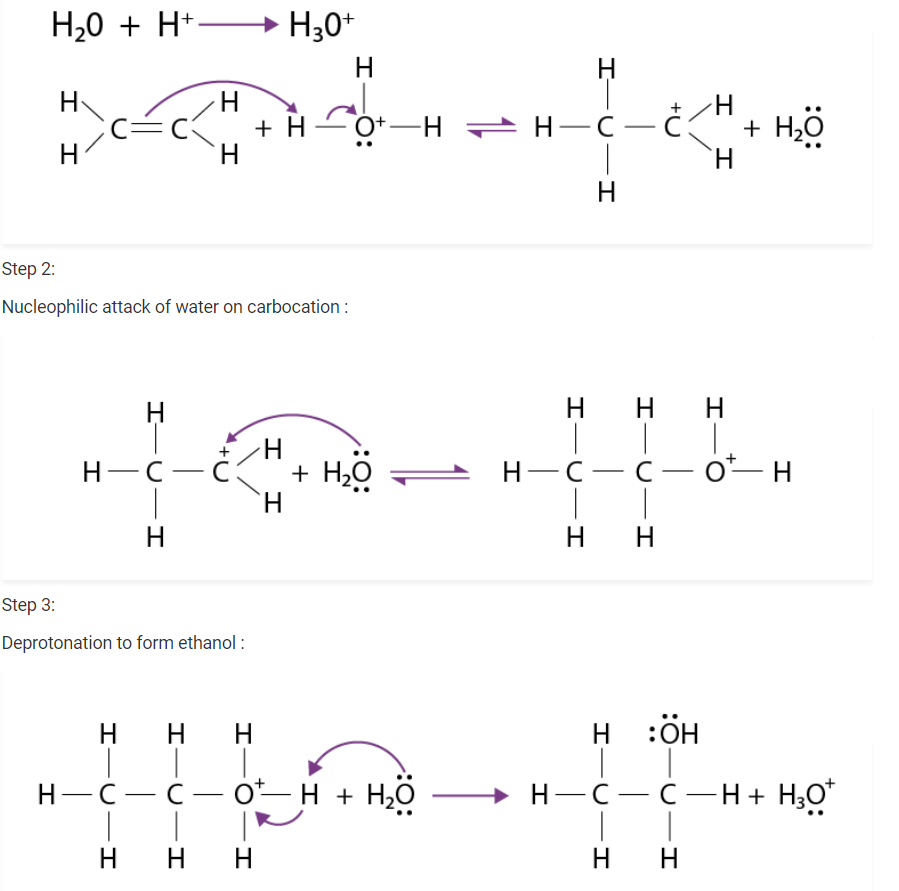
Write the mechanism of hydration of ethene to yield ethanol.
Solution :
There are three steps that are involved in the mechanism of hydration of ethene to form ethanol. these steps are as follows:
Step 1 :
Protonation of ethene to form carbocation by an electrophilic attack of H3 O + :
Q 11.11:
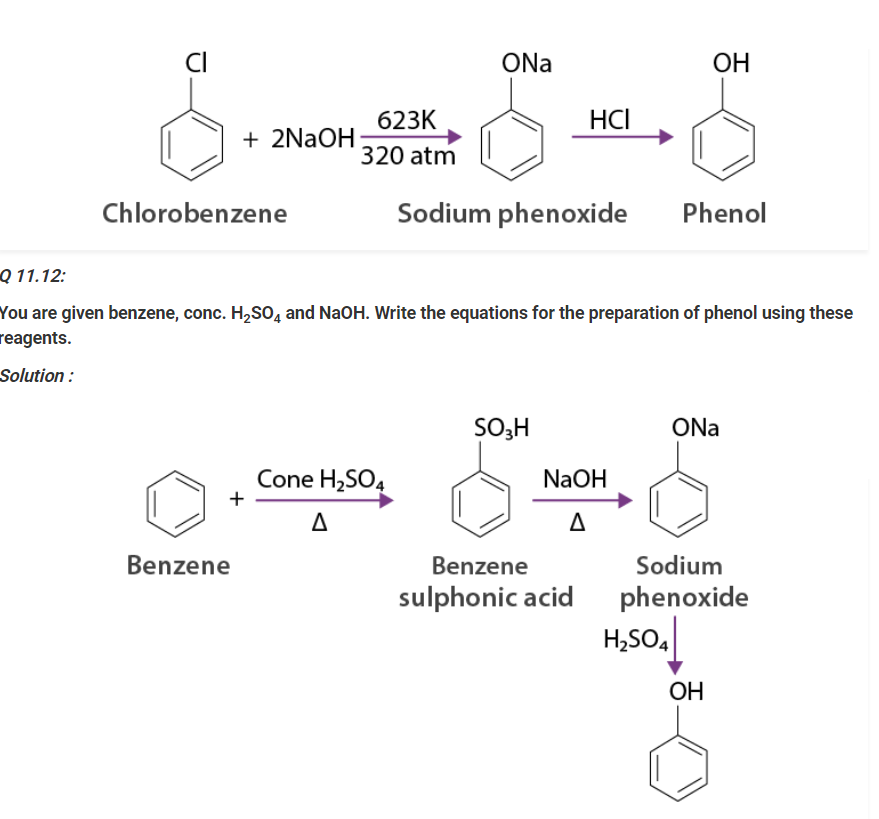
Write a chemical reaction for the preparation of phenol from chlorobenzene.
Solution :
Chlorobenzene is combined with NaOH ( at 623 K & 320 atm pressure ) to prepare sodium phenoxide, resulting in phenol on acidification.
Q 11.13:
Show how will you synthesise:
(i) 1-phenylethanol from a suitable alkene.
(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction.
(iii) pentan-1-ol using a suitable alkyl halide?
Solution :
(i) By acid-catalyzed hydration of ethylbenzene ( styrene ), 1 – phenylethanol can be synthesized.
Q 11.14:
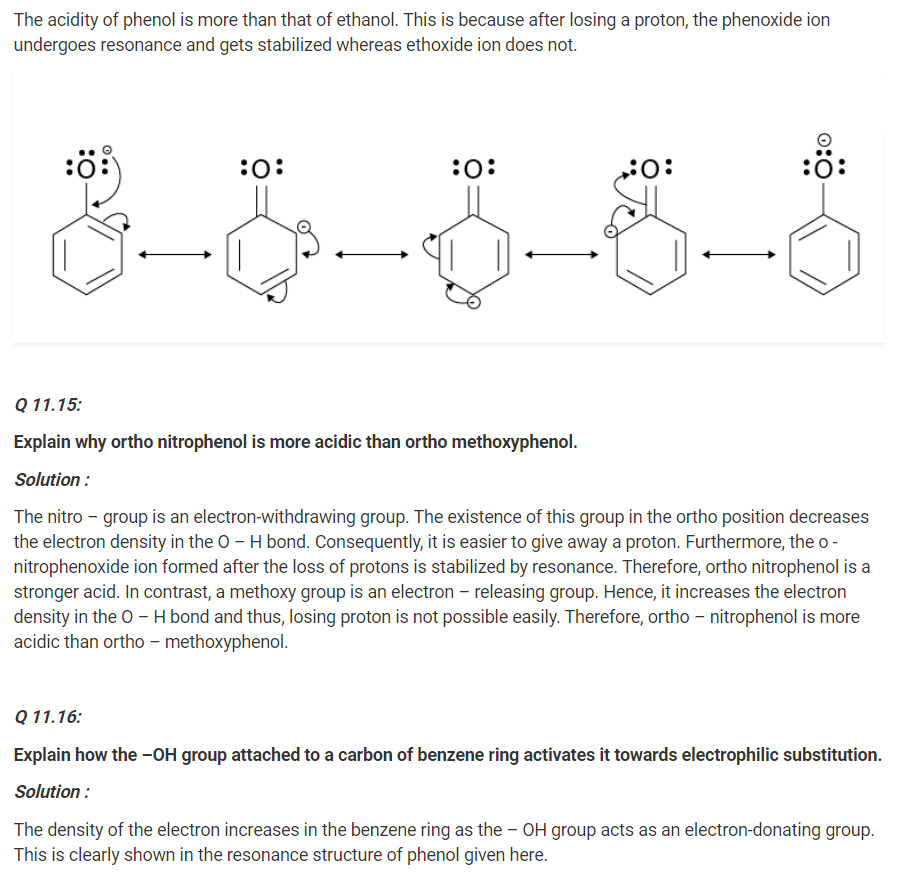
Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Solution :
The acidic nature of phenol can be proven with the two reactions given below :
(i) Phenol reacts with sodium to give sodium phenoxide, liberating H2.
(iv) Unsymmetrical ether:
When an oxygen atom has two groups on two of its side, it is called Unsymmetrical ether. ( i.e., the number of carbon atoms is unequal ). For eg: ethyl methyl ether ( CH 3 – O − CH 2 CH 3 ).
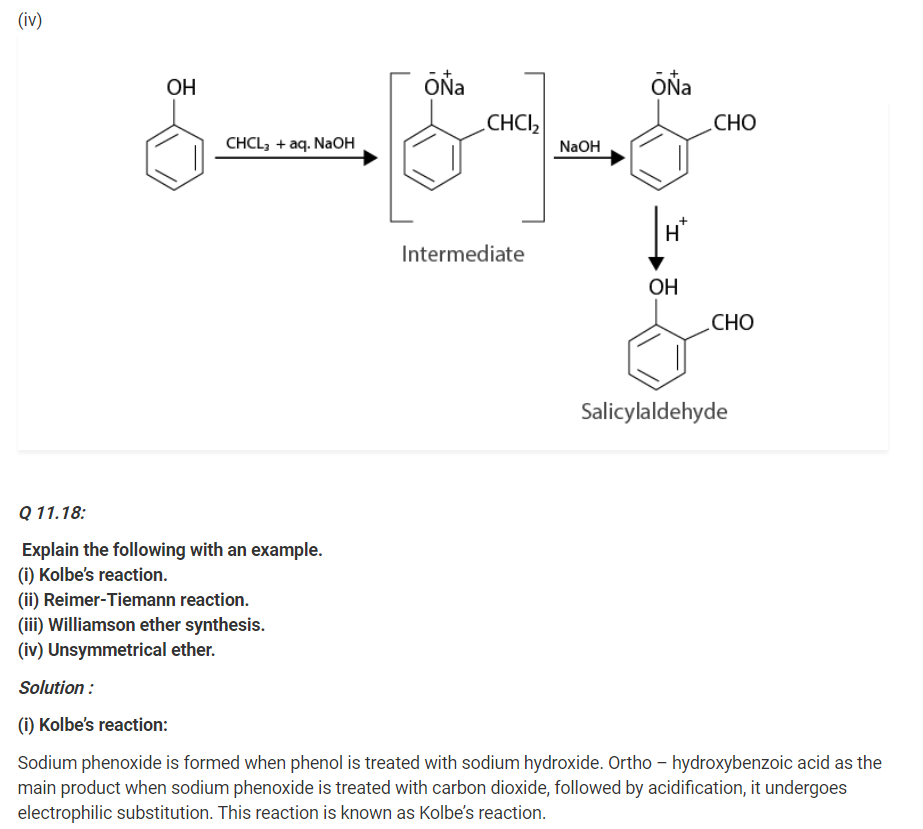
Q 11.19:
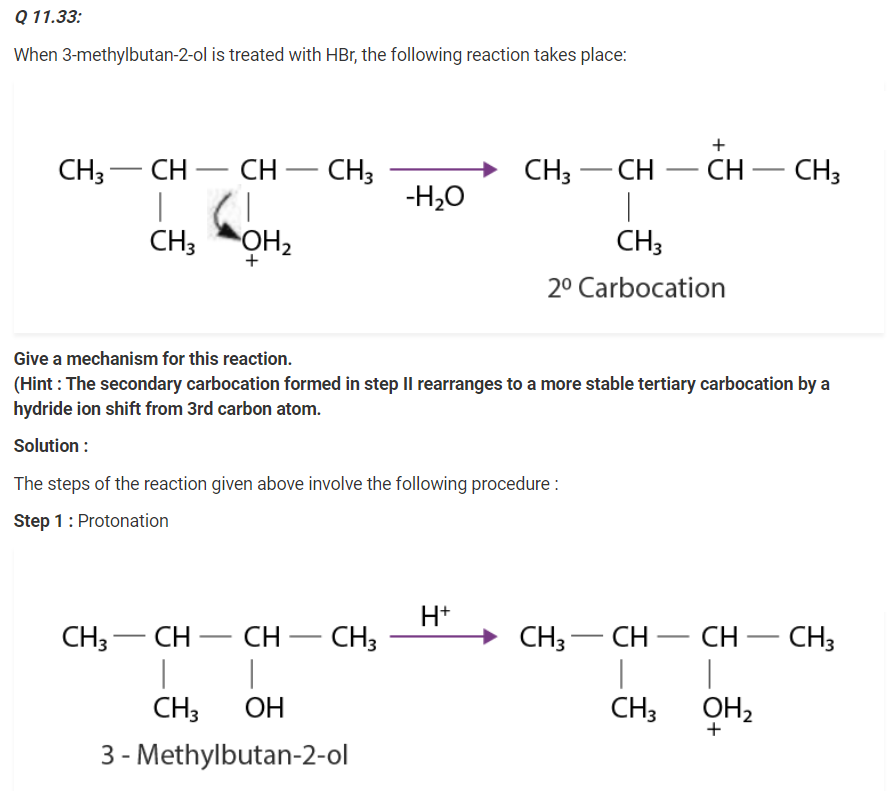
Write the mechanism of acid dehydration of ethanol to yield ethene.
Solution :
The mechanism of acid dehydration of ethanol to yield ethene involves the following three steps :
Step 1 :
Formation of ethyl oxonium by protonation of ethanol :
The acid is released in Step 3 which was being consumed in Step 1. It is removed to shift the equilibrium in a forward direction, after ethene is formed.
Q 11.20:
How are the following conversions carried out?
(i) Propene → Propan-2-ol.
(ii) Benzyl chloride → Benzyl alcohol.
(iii) Ethyl magnesium chloride → Propan-1-ol.
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
Solution :
(i) When propene is reacted with water in the presence of an acid as a catalyst, as a result, we obtain propan – 2 – ol.
Q 11.21:
Name the reagents used in the following reactions:
(i) Oxidation of primary alcohol to a carboxylic acid.
(ii) Oxidation of primary alcohol to aldehyde.
(iii) Bromination of phenol to 2,4,6-tribromophenol.
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-ol to propene.
(vi) Butan-2-one to butan-2-ol.
Solution :
(i) NaBH4 or LiAlH4 to acidified KMnO4
(ii) Pyridinium chlorochromate (PCC)
(iii) Bromine water
(iv) Acidified potassium permanganate
(v) Concentrated H2SO4 or H3PO4
(vi) Catalytic hydrogenation or sodium borohydride (NaBH4) or lithium aluminium hydride (LiAlH4)
Q 11.22:
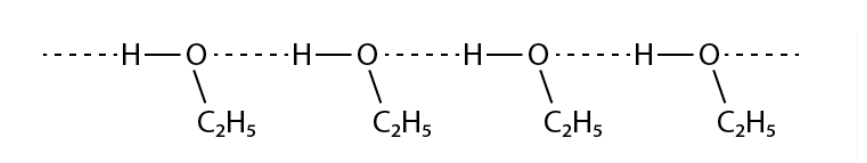
Give a reason for the higher boiling point of ethanol in comparison to methoxymethane.
Solution :
Ethanol experiences intermolecular H – bonding because of the presence of – OH group, which results in the association of molecules. additional energy is necessary to break these hydrogen bonds. Conversely, methoxymethane does not experience H – bonding. Therefore, ethanol has a higher boiling point when compared to methoxymethane
Q 11.23:
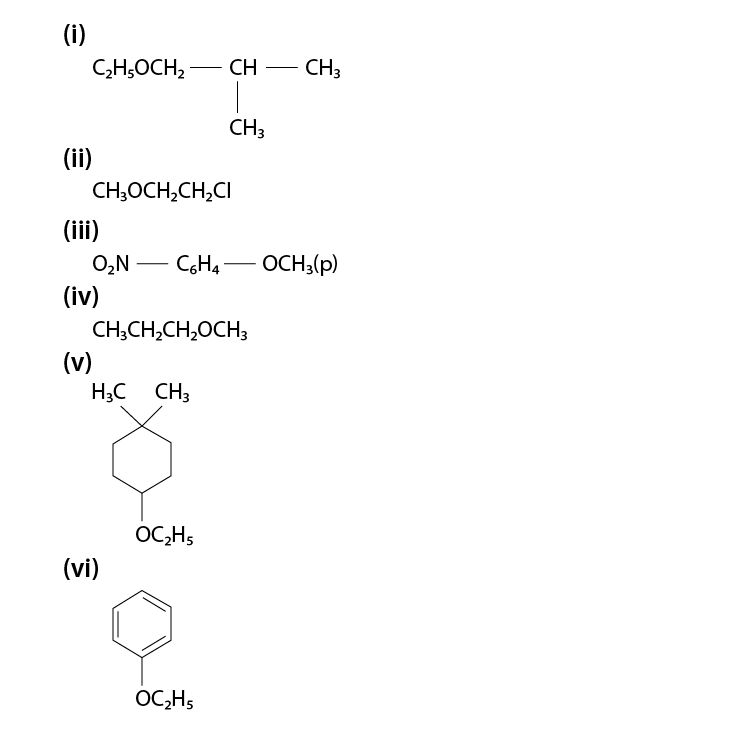
Give IUPAC names of the following ethers:
Solution :
(i) 1 – Ethoxy – 2 – methylpropane
(ii) 2 – Chloro – 1 – methoxyethane
(iii) 4 – Nitroanisole
(iv) 1 – Methoxypropane
(v) 1 – Ethoxy – 4, 4 – dimethylcyclohexane
(vi) Ethoxybenzene
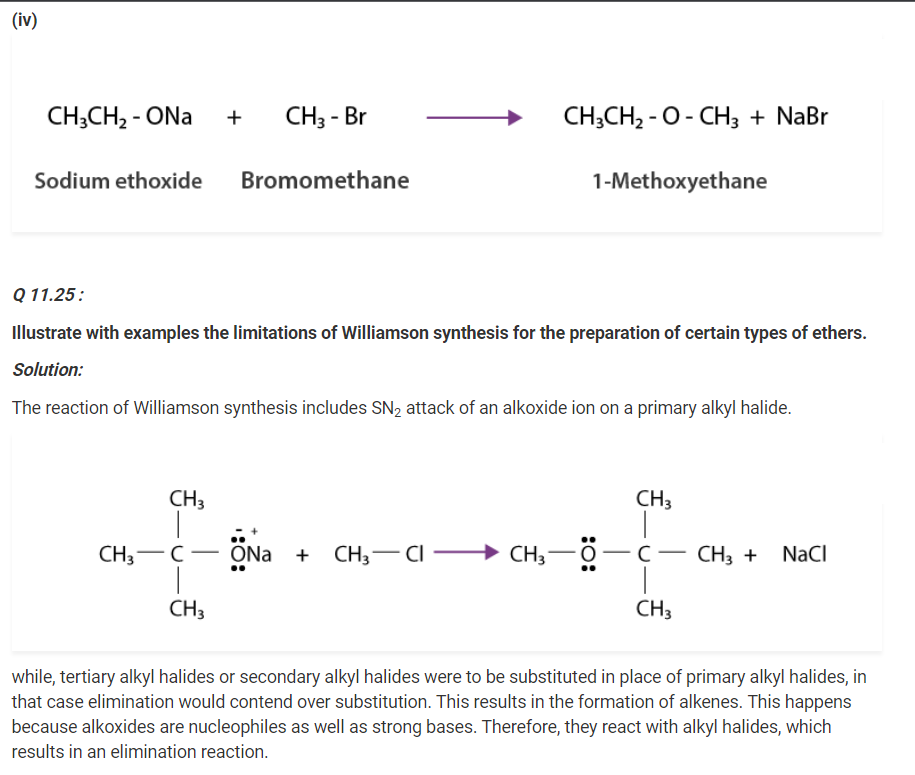
Q 11.24 :
Write the names of reagents and equations for the preparation of the following
ethers by Williamson’s synthesis:
(i) 1-Propoxypropane (ii) Ethoxybenzene
(iii) 2-Methoxy-2-methylpropane (iv) 1-Methoxyethane
Solution :
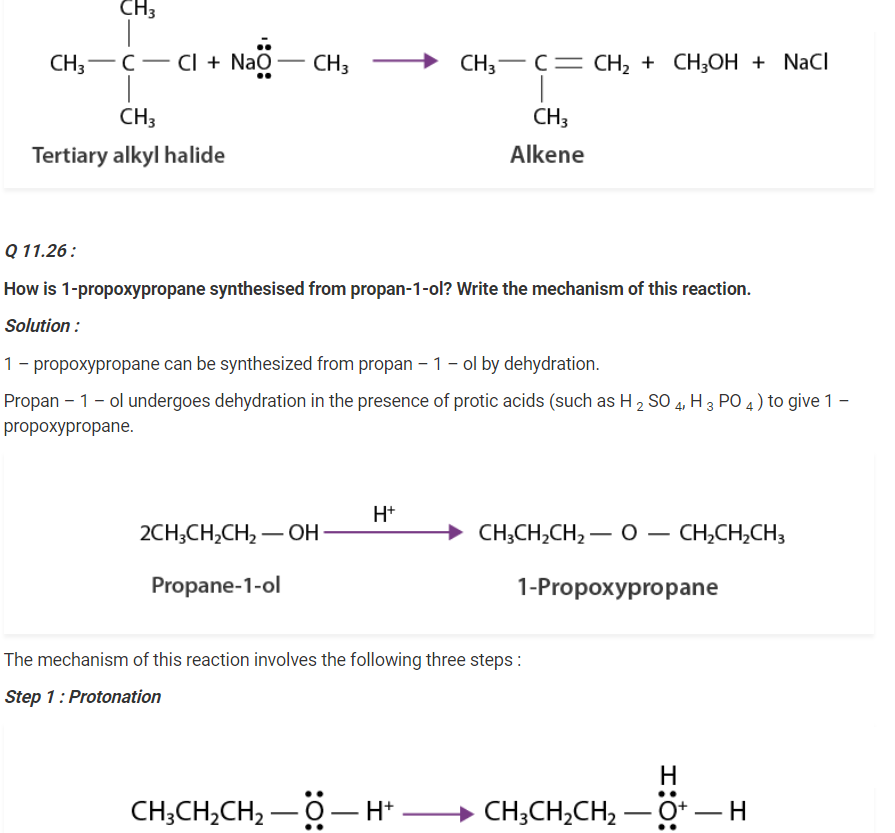
(i)
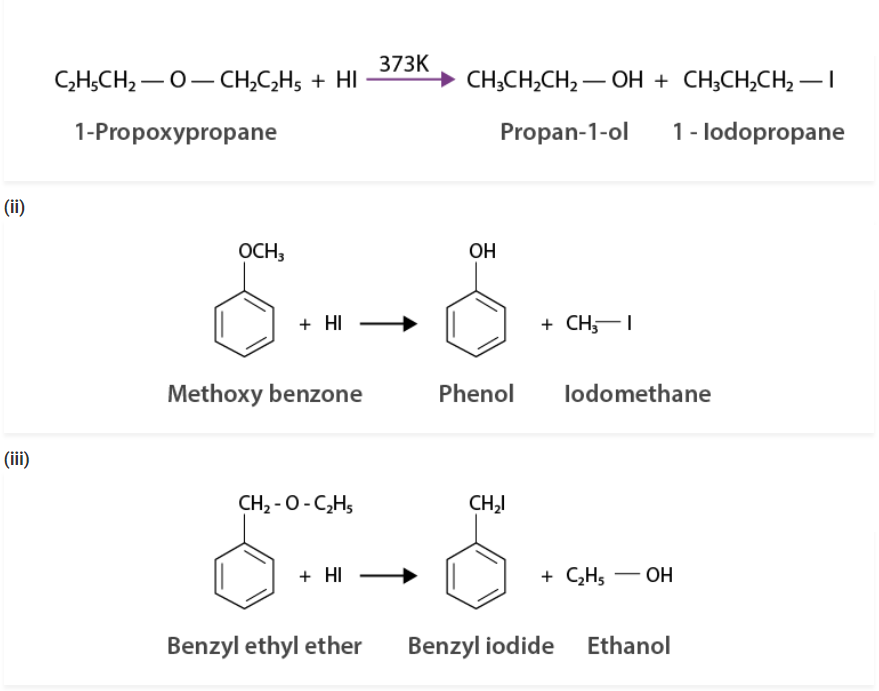
Q 11.28:
Write the equation of the reaction of hydrogen iodide with:
(i) 1-propoxypropane (ii) methoxybenzene and (iii) benzyl ethyl ether
Solution :
Q 11.29:
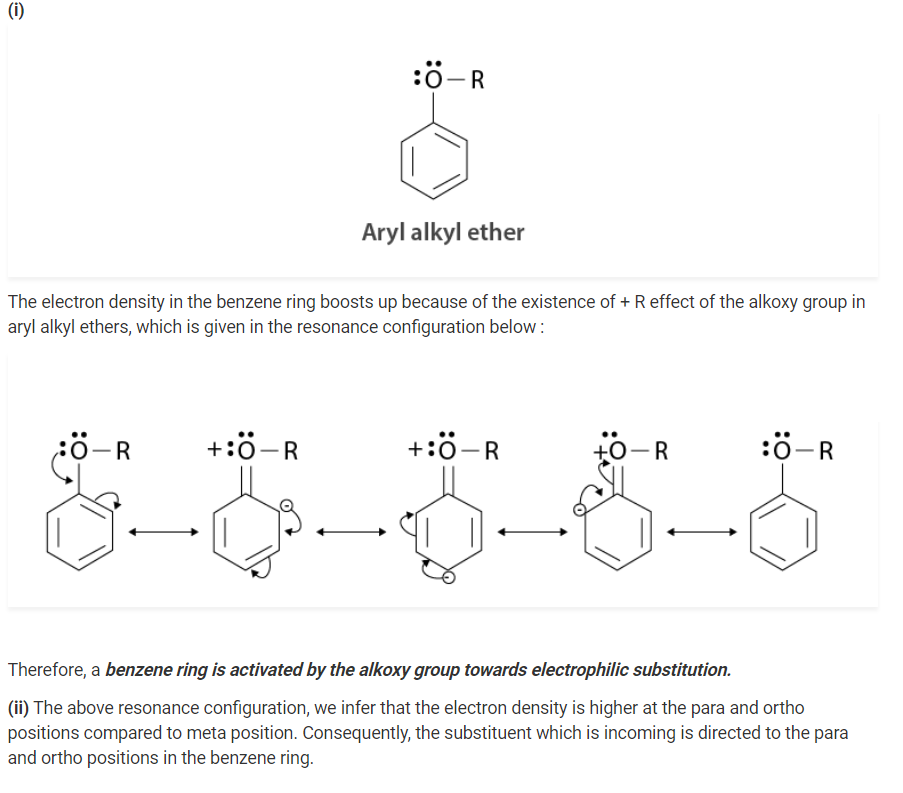
Explain the fact that in aryl alkyl ethers
(i) the alkoxy group activates the benzene ring towards electrophilic substitution
(ii) it directs the incoming substituents to ortho and para positions in the benzene ring
Solution :
Q 11.30:
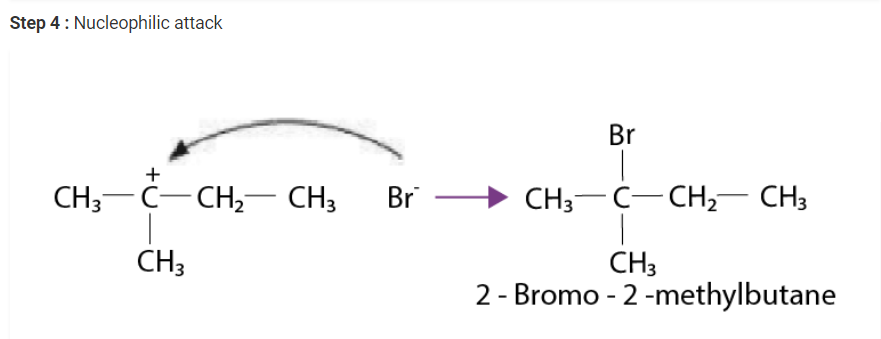
Write the mechanism of the reaction of HI with methoxymethane.
Solution :
The steps of the reaction of methoxymethane with HI are given below :
Step 1 : Protonation of methoxymethane :
Q 11.31:
Write equations of the following reactions:
(i) Friedel-Crafts reaction – alkylation of anisole.
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium.
(iv) Friedel-Craft’s acetylation of anisole.
Solution :
(i)