Electrochemistry:-
It is study of production of electricity from energy which is released during spontaneous chemical reaction and the use of electrical energy to bring about non-spontaneous chemical transformation.
There are two types of cells:-
- Electrochemical Cells (Galvanic Cell)
- Electrolytic Cells
Some Basic Definitions:-
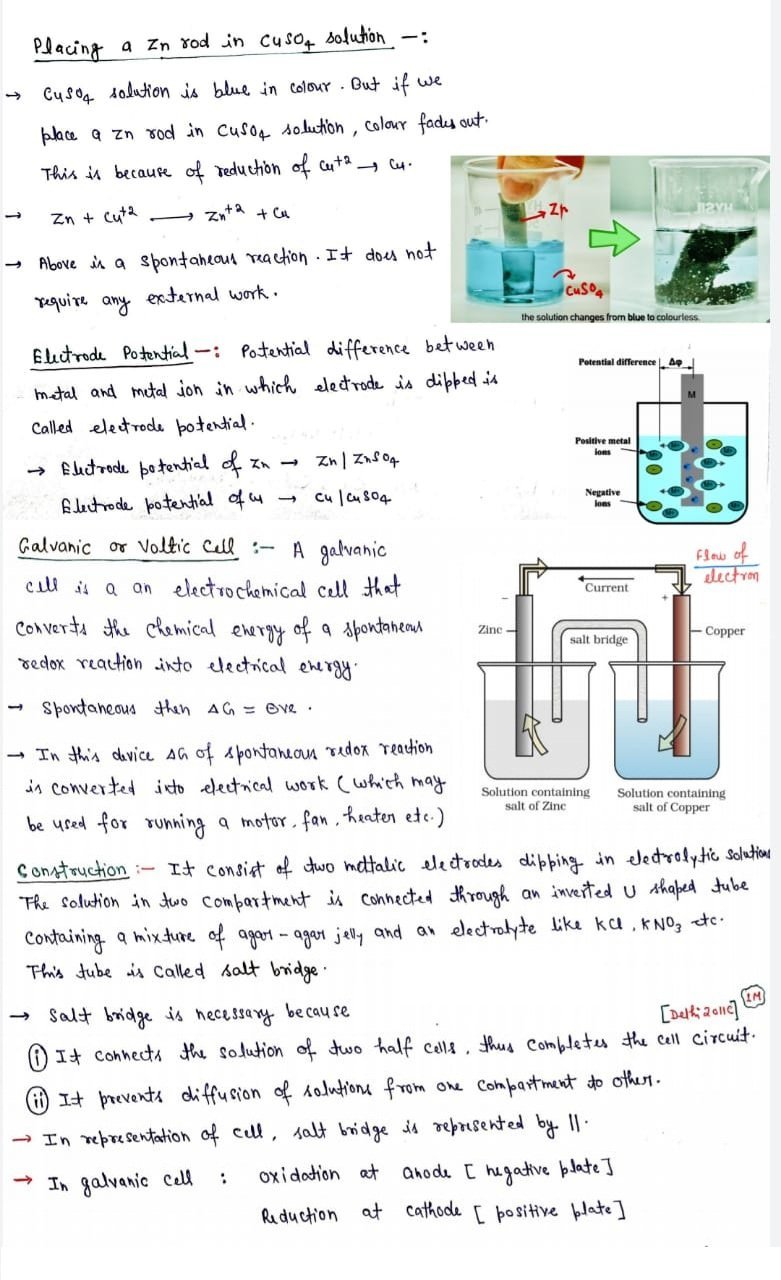
:- Oxidation:- Loss of electron ( Zn ——-> Zn+2 + 2e– )
:- Reduction :- Gain of electron (Cu+2 + 2e– —-> Cu)
:- Electrolyte:- A Solution that contains ions is called electrolyte. Electrolyte is an ionic conductor.
:- Electrode :- Surface at which oxidation or reduction take place.
:- Redox Reaction :- An oxidation-reduction (redox) rection.
Zn + Cu+2 ————> Zn+2 + Cu