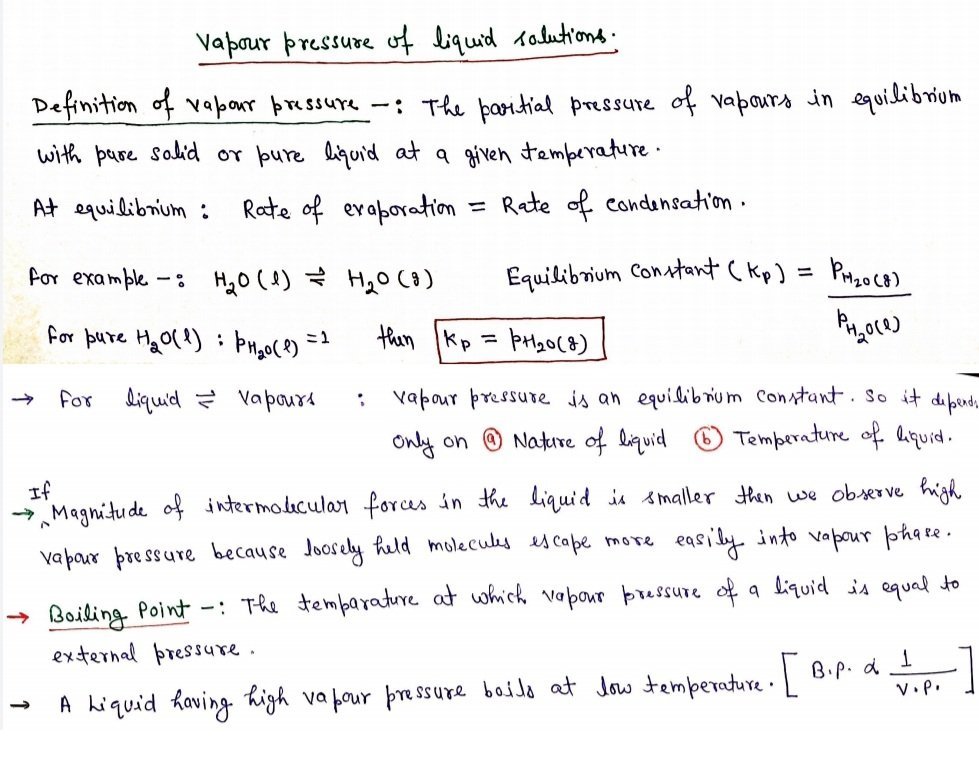
Raoult’s law
- Raoult’s law establishes a quantitative relationship between the partial vapour pressure and mole fraction of a solution.
- This law is only for liquid-liquid solution.
- The law states that for a solution of volatile liquids, the partial vapour pressure (p) of each component in the solution is directly proportional to its mole fraction (x).
- Mathematically, p ∝ x
Or p = p 0x
Where p0 is the vapour pressure of pure component at the same temperature.
- Thus, for component 1, p1 = p1 0 x1 and for component 2 p2 = p20 x
- Let the solvent be represented by 1 and solute be represented by 2. At the point when the solute is non-volatile, just the solvent molecules are present in vapour phase and add to vapour pressure.
Let p1 = vapour pressure of the solvent,
x1 = mole fraction,
pi0 = vapour pressure in the pure state.
According to Raoult’s law
p1 ∝ x1
And p1 = p1 0 x1




p2= Partial vapour pressures of component 2
x1 = Mole fractions of component 1.
x2 = Mole fractions of component 2
- Dalton’s law of partial pressures states that the total pressure (ptotal) of the solution is the sum of the partial pressures of the components of the solution.
- Mathematically, P total = P1 + P2
- Substituting the values of p1 and p2 we get

P1 = y1 Ptotal
P2 = y2 Ptotal
Vapour pressure of solution of solids in liquids
- Solution of salt, sugar or glucose dissolved in water is solutions of solids in liquids.
- Similarly iodine and sulphur dissolved in carbon disulphide are also solutions of solids in liquids
- Addition of anon-volatile solute to a solvent togive a solution results in the exertion of the vapourpressure of the solution uniquely from the solvent.
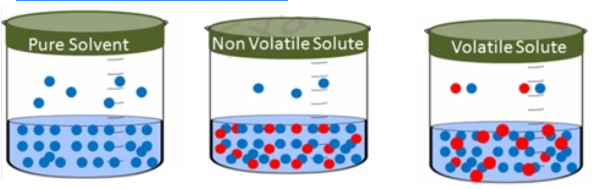
- This vapour pressure is lower than the vapour pressure of the pure solvent at a given temperature.
- The surface of the solution is occupied by both solute andsolvent molecules which results in the reduction of the surface for the solvent molecules. As a result the apparent number ofsolvent molecules escaping from the surface is reduced. This in turn reduces the vapour pressure.
- The reduction of vapour pressure of solvent depends on thequantity of non-volatile solute present in the solution not on its nature.


Positive deviation from Raoult’s Law
- The solute- solute and solvent-solvent interaction are stronger than solute-solvent interaction.
- Let pA = Partial vapour pressures of component A
PB = Partial vapour pressures of component B
Hmix = Change in enthalpy of mixing.
Vmix =Change in volume of mixing.
- pA > pAoxA
- pB > pBoxB
- ΔHmix > 0
- ΔVmix > 0
- Total vapor pressure is more than as expected from Raoult’s law.
- Solution of ethanol and acetonebehaves in the same manner. Molecules of pure ethanol are hydrogen bonded. The molecules of acetone get in between the molecules of ethanol on addition of acetone and break some of the hydrogen bonds between them.

Negative deviation from Raoult’s Law
- The solute-solute and solvent –solvent interactions are weaker than solute-solvent interaction.
- Let pA = Partial vapour pressures of component A
PB = Partial vapour pressures of component B
Hmix = Change in enthalpy of mixing.
Vmix =Change in volume of mixing.
- pA< pAoxA
- pB < pBoxB
- ΔHmix < 0
- ΔVmix < 0
- Total vapor pressure is less than as expected from Raoult’s law.
- Solution of phenol and aniline. It has strong intermolecular hydrogen bonding between phenolicproton and lone pair of nitrogen atom of aniline than the intermolecular hydrogen bonding between similar molecules.
- A solution of chloroform and acetone shows negative deviation from Raoult’s law due to the formation of hydrogen bond with acetone molecule by chloroform molecule. This decreases the chances of molecules of component to escape leading to the decrease in vapour pressure. And hence the solution exhibits negative deviation from Raoult’s law

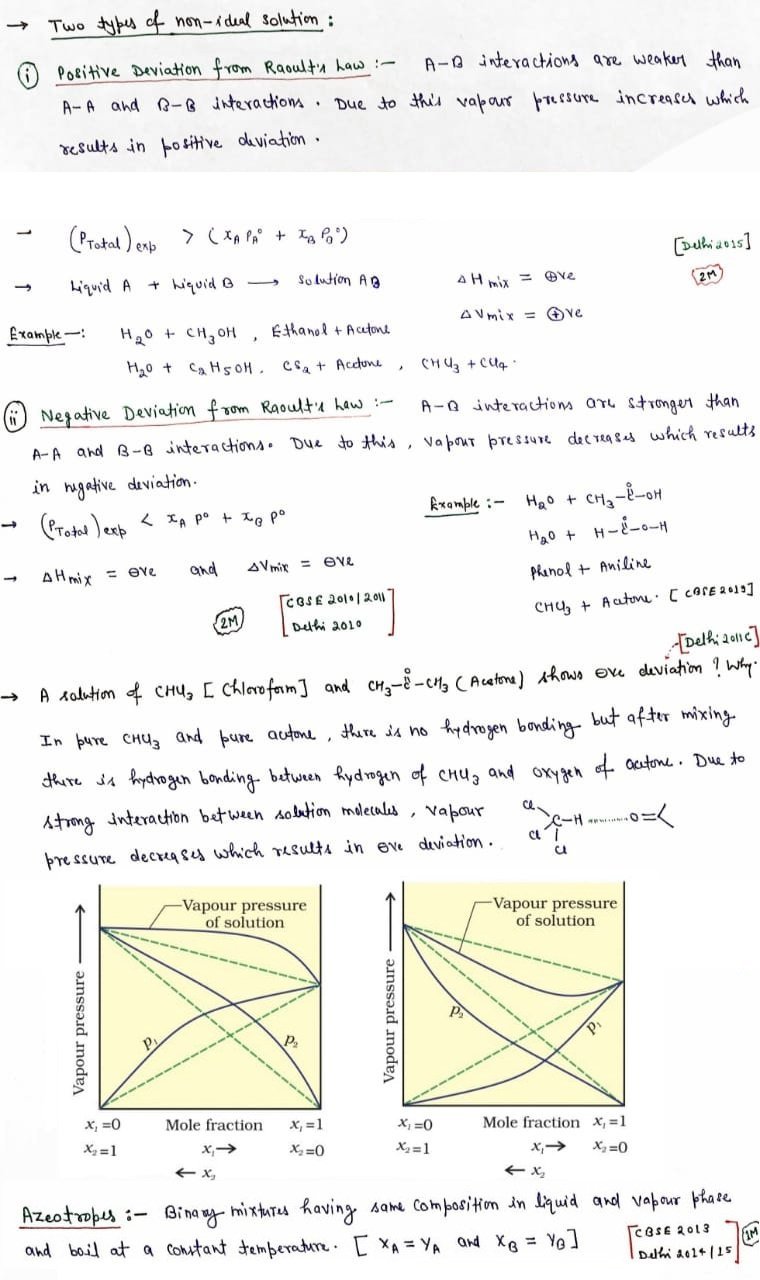
Azeotropes
- Azeotropes are binary mixtures possessing the same synthesis in fluid, vapor stage and boiling stage at a steady temperature.
- The components of azeotropic mixtures are separated by fractional distillation.
- Azeotropes can be broadly categorized into two types-
- Minimum boiling azeotrope
The solutions showing positive deviation from Raoult’s law. For instance, solution of ethanol and water on fractional distillation results in a solution containing approximately 95% by volume of ethanol.
- Maximum boiling azeotrope.
The solutions showing negative deviation from Raoult’s law. For instance, solution of nitric acid and water has the approximate arrangement, 68% nitric corrosive and 32% water by mass, with a breaking point of 393.5 K.

2.14. According to Raoult’s law, what is meant by positive and negative deviaitions and how is the sign of ∆solH related to positive and negative deviations from Raoult’s law?
Sol: Solutions having vapour pressures more than that expected from Raoult’s law are said to exhibit positive deviation. In these solutions solvent – solute interactions are weaker and ∆solH is positive because stronger A – A or B – B interactions are replaced by weaker A – B interactions. Breaking of the stronger interactions requires more energy & less energy is released on formation of weaker interactions. So overall ∆sol H is positive. Similarly ∆solV is positive i.e. the volume of solution is some what more than sum of volumes of solvent and solute.
So there is expansion in volume on solution formation.
Similarly in case of solutions exhibiting negative deviations, A – B interactions are stronger than A-A&B-B. So weaker interactions are replaced by stronger interactions so , there is release of energy i.e. ∆sol H is negative.




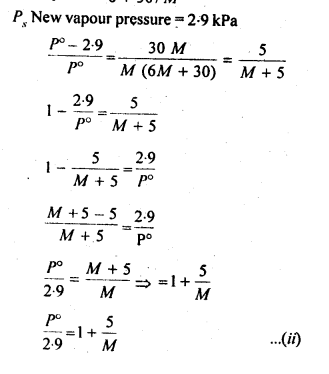

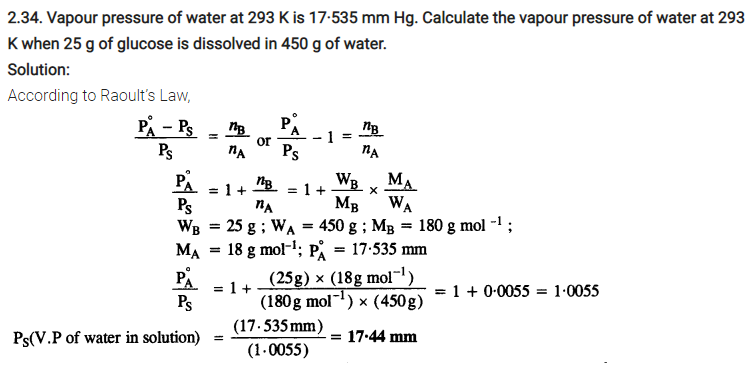
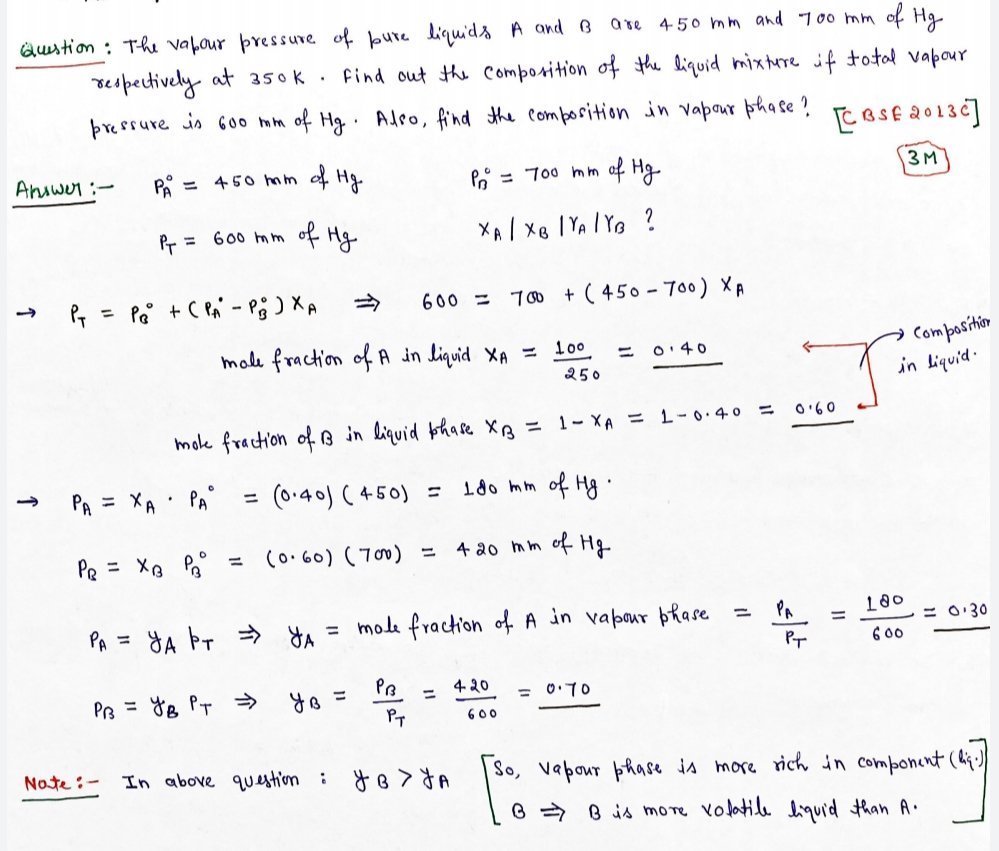
2.36. 100g of liquid A (molar mass 140 g mol-1) was dissolved in 1000g of liquid B (molar mass 180g mol-1). The vapour pressure of pure liquid B was found to be 500 torr. Calculate the vapour pressure of pure liquid A and its vapour pressure in the solution if the total vapour pressure of the solution is 475 torr.

Vapour pressure of pure acetone and chloroform at 328 K are 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot ptotal‘ pchloroform‘ and pacetoneas a function of xacetone. The experimental data observed for different compositions of mixture is.
|
100 ×xacetone |
0 | 11.8 | 23.4 | 36.0 | 50.8 | 58.2 | 64.5 | 72.1 |
|
pacetone /mm Hg |
0 | 54.9 | 110.1 | 202.4 | 322.7 | 405.9 | 454.1 | 521.1 |
|
pchloroform/mm Hg |
632.8 | 548.1 | 469.4 | 359.7 | 257.7 | 193.6 | 161.2 |
120.7 |
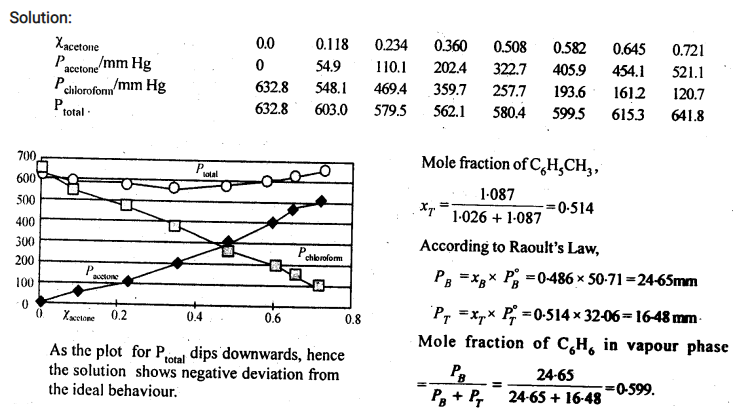
Q. Define Azeotrpes ? [CBSE 2013/Delhi 2014/15]
Q. What is Minimum Boiling Point ? [Delhi 2015]
Q. What is Maximum Boiling Point ? [Delhi 2016]
Q. State the condition for Negative Deviation from Raoult’s Law? [Delhi 2010]
Q. Which type of deviation shown by chloroform and acetone ? [Delhi 2011c]
Q. State the condition of Positive Deviation ? [Delhi2015]
Q. What is the Difference between Ideal and Non-Ideal Solution ? [Delhi 2019/delhi 2017/2012/2013/2014]
Q. How Raoult’s law relates with Henry’s Law? [CBSE 2013/Delhi 2014]
Q. Define Raoult’s law and Plot its graph? [CBSE 2011/2013/Delhi 2011/2010c/2019/2014]