Introduction to Chemical Reactions and Equations
Physical and chemical changes Chemical change – one or more new substances with new physical and chemical properties are formed.
Here, when copper sulphate reacts with iron, two new substances, i.e., ferrous sulphate and copper are formed.

Physical change – change in colour or state occurs but no new substance is formed.
Example: Water changes to steam on boiling but no new substance is formed(Even though steam and water look different when they are made to react with a piece of Na, they react the same way and give the exact same products). This involves only a change in state (liquid to vapour).
Observations that help determine a chemical reaction
A chemical reaction can be determined with the help of any of the following observations:
a) Evolution of a gas
b) Change in temperature
c) Formation of a precipitate
d) Change in colour
e) Change of state
Chemical reaction
Chemical reactions are chemical changes in which reactants transform into products by making or breaking of bonds (or both) between different atoms.
Types of chemical reactions
Taking into consideration different factors, chemical reactions are grouped into multiple categories.
Few examples are:
● Combination
● Decomposition
● Single Displacement
● Double displacement
● Redox
● Endothermic
● Exothermic
● Precipitation
● Neutralisation
Chemical Reactions and Equations I
Word equation
A word equation is a chemical reaction expressed in words rather than chemical formulas. It helps identify the reactants and products in a chemical reaction.
For example,
Sodium + Chlorine → Sodium chloride
The above equation means: “Sodium reacts with chlorine to form sodium chloride.”
Symbols of elements and their valencies
A symbol is the chemical code for an element. Each element has one or two-letter atomic symbol, which is the abbreviated form of its name.
Valency is the combining capacity of an element. It can be considered as the number of electrons lost, gain or shared by an atom when it combines with another atom to form a molecule.
Writing chemical equations
Representation of a chemical reaction in terms of symbols and chemical formulae of the reactants and products is known as a chemical equation.
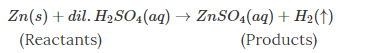
• For solids, the symbol is “(s)”.
• For liquids, it is “(l)”.
• For gases, it is “(g)”.
• For aqueous solutions, it is “(aq)”.
• For gas produced in the reaction, it is represented by “(↑)”.
• For precipitate formed in the reaction, it is represented by “(↓)”.
Balancing of a Chemical Reaction
Conservation of mass
According to the law of conservation of mass, no atoms can be created or destroyed in a chemical reaction, so the number of atoms for each element in the reactants side has to balance the number of atoms that are present in the products side.
In other words, the total mass of the products formed in a chemical reaction is equal to the total mass of the reactants participated in a chemical reaction.
Balanced chemical equation
The chemical equation in which the number of atoms of each element in the reactants side is equal to that of the products side is called a balanced chemical equation.
Steps for balancing chemical equations
Hit and trial method: While balancing the equation, change the coefficients (the numbers in front of the compound or molecule) so that the number of atoms of each element is same on each side of the chemical equation.
Short-cut technique for balancing a chemical equation
Example:
aCaCO3 + bH3PO4 → cCa3(PO4)2 + dH2CO3
Set up a series of simultaneous equations, one for each element.
Ca: a=3c
C: a=d
O: 3a+4b=8c+3d
H: 3b=2d
P: b=2c
Let’s set c=1
Then a=3 and
d = a = 3
b = 2c = 2
So a=3; b=2; c=1; d=3
The balanced equation is
3CaCO3 + 2H3PO4 → Ca3 (PO4)2 + 3H2CO3
Chemical Reactions and Equations II
Types of chemical reactions
Taking into consideration different factors, chemical reactions are grouped into multiple categories.
Few examples are:
● Combination
● Decomposition
● Single Displacement
● Double displacement
● Redox
● Endothermic
● Exothermic
● Precipitation
● Neutralisation
Combination reaction In a combination reaction, two elements or one element and one compound or two compounds combine to give one single product.

Decomposition reaction
A single reactant decomposes on the application of heat or light or electricity to give two or more products.
Types of decomposition reactions:
a. Decomposition reactions which require heat – thermolytic decomposition or thermolysis.

Thermal decomposition of HgO
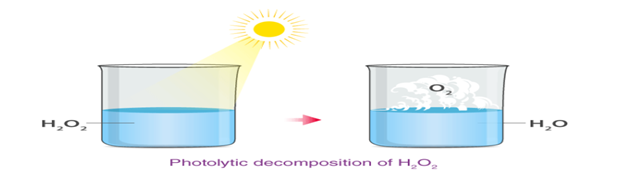
b. Decomposition reactions which require light – photolytic decomposition or photolysis.
Photolytic decomposition of H2O2
c. Decomposition reactions which require electricity – electrolytic decomposition or electrolysis.
Electrolytic decomposition of H2O
Displacement reaction
More reactive element displaces a less reactive element from its compound or solution.

Double displacement reaction An exchange of ions between the reactants takes place to give new products.
For example,
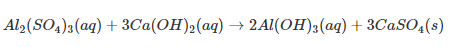
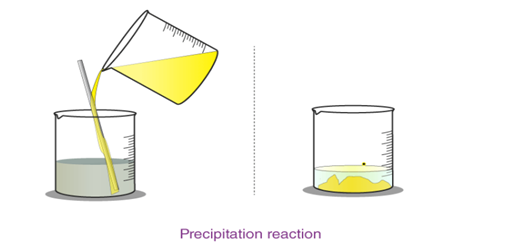
Precipitation reaction An insoluble compound called precipitate forms when two solutions containing soluble salts are combined.

Redox reaction
Oxidation and reduction take place simultaneously.
Oxidation: Substance loses electrons or gains oxygen or loses hydrogen.
Reduction: Substance gains electrons or loses oxygen or gains hydrogen.
Oxidising agent – a substance that oxidises another substance and self-gets reduced.
Reducing agent – a substance that reduces another substance and self-gets oxidised.

Endothermic and exothermic reaction
Exothermic reaction – heat is evolved during a reaction. Most of the combination reactions are exothermic.
Al + Fe2O3 → Al2O3 + Fe + heat
CH4 + 2O2 → CO2 + 2H2O + heat
Endothermic – Heat is required to carry out the reaction.
6CO2 + 6H2O + Sunlight → C6H12O6 + 6O2
Glucose
Most of the decomposition reactions are endothermic.
Corrosion
Gradual deterioration of a material, usually a metal, by the action of moisture, air or chemicals in the surrounding environment.
Rusting:
4Fe(s) + 3O2(from air) + xH2O(moisture) → 2Fe2O3.xH2O(rust)
Corrosion of copper:
Cu(s) + H2O(moisture) + CO2(from air) → CuCO3.Cu(OH)2(green)
Corrosion of silver:
Ag(s) + H2S (from air) → Ag2S(black) + H2(g)
Rancidity
It refers to the oxidation of fats and oils in food that is kept for a long time. It gives foul smell and bad taste to food. Rancid food causes stomach infection on consumption.
Prevention:
(i) Use of air-tight containers
(ii) Packaging with nitrogen
(iii) Refrigeration
(iv) Addition of antioxidants or preservatives
Class 10th science