Introduction
What is Kinetic Theory?
- Kinetic theory explains the behaviour of gases based on the idea that the gas consists of rapidly moving atoms or molecules.
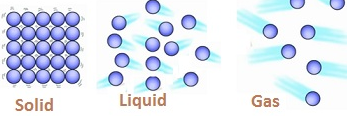
- In solids the molecules are very tightly packed as inter molecular space is not present In liquids inter molecular spaces are more as compared to solids and in gases the molecules are very loosely packed as intermolecular spaces are very large.
- The random movement of molecules in a gas is explained by kinetic theory of gases.
- We will also see that why kinetic theory is accepted as a success theory.
- Kinetic theory explains the following:-
- Molecular interpretation of pressure and temperature can be explained.
- It is consistent with gas lawsand Avogadro’s hypothesis.
- Correctly explains specific heat capacities of many gases.
Molecular nature of matter

John Dalton
- Atomic hypothesis was given by many scientists. According to which everything in this universe is made up of atoms.
- Atoms are little particles that move around in a perpetual order attracting each other when they are little distance apart.
- But if they are forced very close to each other then they rebel.
- For example: – Consider a block of gold. It consists of molecules which are constantly moving.
- Dalton’s atomic theory is also referred as the molecular theory of matter.This theory proves that matter is made up of molecules which in turn are made up of atoms.
- According to Gay Lussac’s law when gases combine chemically to yield another gas,their volumes are in ratios of small integers.
- Avogadro’s law states that the equal volumes of all gases at equal temperature and pressure have the same number of molecules.
- Conclusion: – All these laws proved the molecular nature of gases.
- Dalton’s molecular theory forms the basis of Kinetic theory.
Why was Dalton’s theory a success?
- Matter is made up of molecules, which in turn are made up of atoms.
- Atomic structure can be viewed by an electron microscope.
Solids, Liquids, Gases in terms of molecular structure
| Basis of Difference | Solids | Liquids | Gases |
| Inter Atomic Distance(distance between molecules). | Molecules are very tightly packed. Inter atomic distance is minimum. | Molecules are not so tightly packed. Inter atomic distance is more as compared to solids. | Molecules are loosely packed .Free to move. Inter atomic distance is maximum. |
| Mean Free Path is the average distance a molecule can travel without colliding. | No mean free path. | Less mean free path. | There is mean free path followed by the molecules. |
Behaviour of Gases
- Gases at low pressures and high temperatures much above that at which they liquify (or solidify) approximately satisfy a relation between their pressure, temperature and volume:
- PV=KT (i)
- This is the universal relation which is satisfied by all gases.
- where P, V, T are pressure,volume and temperature resp. and
- K is the constant for a given volume of gas. It varies with volume of gas.
- K=NkB where
- N=number of molecules and
- kB = Boltzmann Constant and its value never change.
- From equation (i) PV= NkB
- Therefore PV/NT = constant=(kB)(Same for all gases).
- Consider there are 2 gases :- (P1,V1,T1) and (P2, V2,T2) where P, V and T are pressure, volume and temperature resp.
- Therefore P1,V1/(N1T1) = P2V2/(N2T2)
- Conclusion: – This relation is satisfied by all gases at low pressure and high temperature.


= (11.143×105x30x10-3)/ (8.314×290)
= 13.86
But, n2 = m2/M Where, m2 is the mass of oxygen
remaining in the cylinder m2 = n2M = 13.86 × 32
= 453.1 g
The mass of oxygen taken out of the cylinder is given by the relation:
Initial mass of oxygen in the cylinder – Final mass of oxygen in the cylinder
= m1 – m2
= 584.84 g – 453.1 g
= 131.74 g
= 0.131 kg
Therefore, 0.131 kg of oxygen is taken out of the cylinder.
Problem:- An air bubble of volume 1.0 cm3 rises from the bottom of a lake 40 m deep at a temperature of 12 °C. To what volume does it grow when it reaches the surface, which is at a temperature of 35 °C?
Answer:- Volume of the air bubble, V1 = 1.0 cm3 = 1.0 × 10–6 m3
Bubble rises to height, d = 40 m
Temperature at a depth of 40 m, T1 = 12°C = 285 K
Temperature at the surface of the lake, T2 = 35°C = 308 K
The pressure on the surface of the lake:
P2 = 1 atm = 1 ×1.013 × 105 Pa
The pressure at the depth of 40 m:
P1 = 1 atm + dρg Where, ρ is the density ofwater = 103 kg/m3 g is the acceleration due
to gravity = 9.8 m/s2
Therefore, P1 = 1.013 × 105 + 40 × 103 × 9.8 = 493300 Pa
We have: P1 V1/T1 = P2V2/T2
Where, V2 is the volume of the air bubble when it reaches the surface
V2 = P1 V1T2/T1P2
= (493300) (1.0×10-6)308/(285×1.013×105)
= 5.263 × 10–6 m3 or 5.263 cm3
Therefore, when the air bubble reaches the surface, its volume becomes 5.263 cm3.
Justification of the Avogadro’s hypothesis from equation of gas
- Avogadro’s hypothesis states that equal volumes of all gases at equal temperature and pressure have the same number of molecules.
- Consider the equation PV/NT = constant and if P,V and T are same for 2 gases then N(number of molecules) is also same.
- According to Avogadro’s hypothesis number of molecules per unit volume is same for all gasesat a fixed P and T.
- Avogadro number is denoted by NA. WhereA denotes Avogadro number.
- NA = 6.02×1023. It is universal value.
- Experimentally it has been found that the mass of 22.4 litres of any gas is equal to molecular weight in grams at standard temperature and pressure.
