About Lesson
Zeroth Law of Thermodynamics
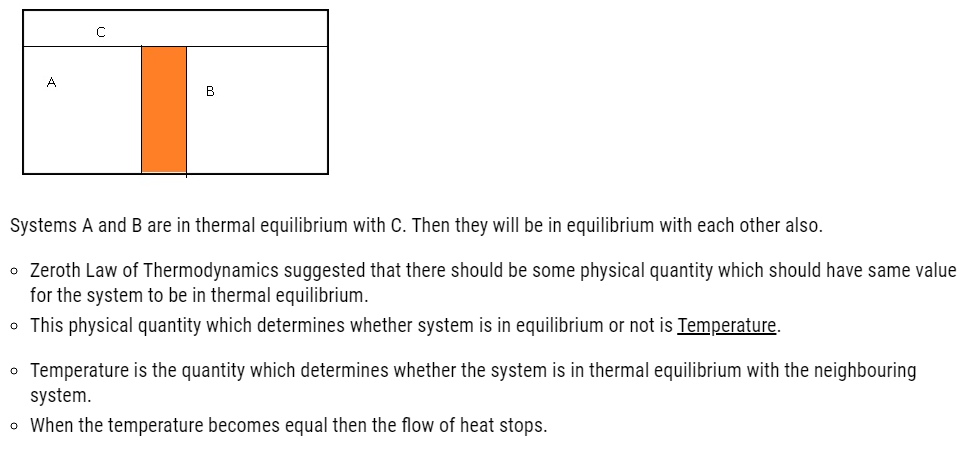
- Zeroth law of thermodynamics states that when two systems are in thermal equilibrium through a third system separately then they are in thermal equilibrium with each other also.
- For eg: – Consider two systems A and B which are separated by an adiabatic wall. Heat flow happens between systems A and C, and between B and C, due to which all 3 systems attain thermal equilibrium.
Thermodynamic state variables
Thermodynamic state variables are the macroscopic quantities which determine the thermodynamic equilibrium state of a system.
- These macroscopic quantities are known as thermodynamics state variables.
- Since these macroscopic quantities describe the behaviour of thermodynamic system it is known as thermodynamic.
- As they determine the state of the system that is pressure, volume and temperature, at one particular time they are known as thermodynamic state variables.
- Pressures (P), Volume (V), Temperature (T), mass (m), Internal energy (U) are the thermodynamic state variables.
- These variables can tell us the position or the condition of any gas at that particular time.
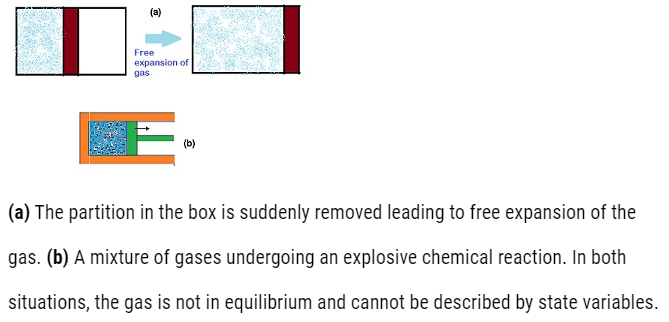
- A system not in equilibrium cannot be described by state variables. It means the macroscopic variables are changing with time and they are not constant.
Types of thermodynamic state variables:-
- Extensive variables: – They indicate the size of the system, which means extensive variables are those that depend on the mass of the system or the number of particles in the system. Example: volume, mass, internal energy. If we consider a system whose mass is greater than the size of that system is greater. All these depend on the size of the system.
- Intensive variables: –
- They don’t depend on size of the system.
- Pressure and Temperature are known as intensive variables.
Equation of State
- Equation of State depicts the relationship between the state variables (pressure, mass, volume, density).
- It describes about the state of the matter under given set of physical conditions.
- Consider an ideal gas the equation of state is
PV= μRT where
- P, V and T are state variables and μ = number of moles
- For a fixed amount of gas we can have two independent variables either volume and temperature or volume and pressure.
- All the variables cannot be independent at the same time.
- This is known equation of state for ideal gas.
Internal Energy
- It is defined as the sum of kinetic energies and potential energies of the molecules constituting the system as a whole and not of individual molecule.
- It is macroscopic variable of the system.
- It is denoted by U.
- It is thermodynamic state variable.
- It is an extensive variable as it depends on the size of the system.
- It can be specified by values of pressure, volume and temperature at that particular time.
- It only depends on the state of the system at that particular time.


Work: – We can do some work on the system (in this case system is bottle with a balloon tied) as a result the internal energy of the system changes.
Internal energy increases when the system absorbs heat and some work is done on the system similarly internal energy decreases if we change the conditions.
How Internal energy is different from Work and Heat
- Heat and work are not state variables unlike internal energy.
- They are modes of energy transfer to system resulting in change in internal energy.