Perfect Gas Equation
- Perfect gas equation is given by PV=μRT,
- WhereP,V are pressure, volume, T =absolute temperature,μ = number of moles and R =universal gas constant.
- R= kBNA where kB = Boltzmann constant and NA = Avogadro’s Number
- This equation tells about the behaviour of gas at a particular situation.
- If a gas satisfies this equation then the gas is known as Perfect gas or an ideal gas.
Different Forms of Perfect Gas Equation
- PV=μRT (i)
- Where μ (no. of moles) = N/NA where N=no of molecules and NA = Avogadro number(no of molecules in 1 mole of gas).Orμ = M/Mowhere M=mass of sample of gas and Mo = molar mass.
- PV = (N/NA)RT(putting μ=N/NA in equation(i))
- By simplifying PV = NkBT
- PV=NkBT => P = (N/V) kBT => P=nkBT where n(number density) =N/V where N=number of molecules and V=volume.
- Therefore we get PV=nkBT
- Substitute μ = M/Mo in equation(i)
- PV=(M/Mo) RT => P=(M/V)1/MoRT where
- ρ(mass density of the gas) = M/V
- Therefore P=ρRT/Mo
Ideal Gas
- A gas that satisfies the perfectgas equation exactly at all pressures and temperatures.
- Ideal gas is atheoretical concept.
- No real gas is truly ideal.A gas which is ideal is known as real gas.
- Real gases approach the ideal gas behaviour for low pressures and high temperatures.
Problem:- ( Kinetic Theory Notes )
Estimate the total number of air molecules (inclusive of oxygen, nitrogen, water vapour
and other constituents) in a room of capacity 25.0 m3 at a temperature of 27 °C and 1 atm
pressure.
Answer:-
Volume of the room, V = 25.0 m3
Temperature of the room, T = 27°C = 300 K
Pressure in the room, P = 1 atm = 1 × 1.013 × 105 Pa
The ideal gas equation relating pressure (P), Volume (V), and absolute temperature (T)
can be written as:
PV = kBNT
Where,
KB is Boltzmann constant = 1.38 × 10–23 m2 kg s–2 K–1
N is the number of air molecules in the room
N=PV/ KBT
=1.013×105x25/1.38×10-23x300
= 6.11 × 1026 molecules
Therefore, the total number of air molecules in the given room is 6.11 × 1026
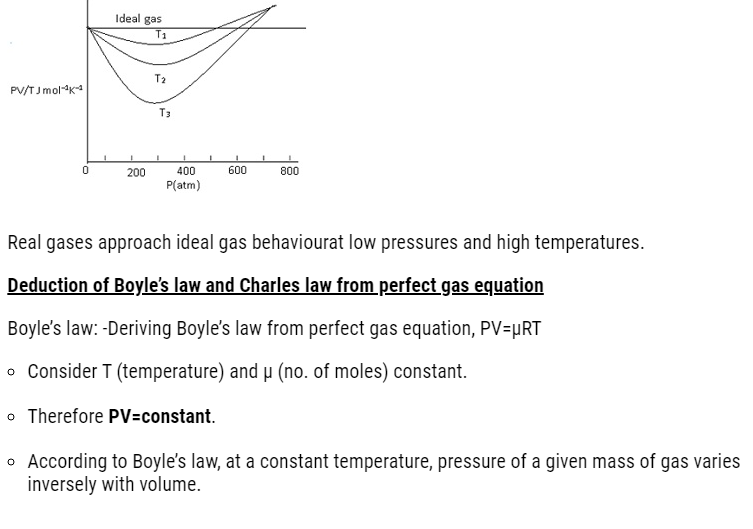
Real gases deviation from ideal gas
- Real gases approach the ideal gas behaviour for low pressures and high temperatures.
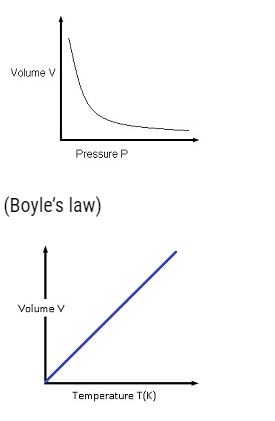
- Ideal gas equation PV=μRT, for 1 mole ,μ=1,PV=RT
- =>PV/RT=constant
- Graph should be a straight line(parallel to x-axis) for ideal gas.
- This means it has constant value at all temperature and all pressure.
But in case of real gases graph approach ideal gas behaviour at high temperature and low pressure.
- At high temperature and low pressure molecules are far apart. When temperature is increased the molecules will move randomly far from each other.
- As a result molecular interaction decreases the gas behaves as an ideal gas.
- The ideal behaviour comes into picture when the molecular present inside the gas don’t interact with each other.
Charles’s law:-Consider If P(Pressure) is constant, then