Hydrogen bonding
Hydrogen bond
Hydrogen bond is formed when hydrogen is attached to some electronegative element like O, N and F. It forma a special bond with them called as hydrogen bond.
Types of Hydrogen bond
- Intermolecular H bond.
- Intra-molecular H bond.
Intermolecular Hydrogen bond: It is a bond formed between two different molecules.
Example: H-F—–H-F—-H-F
Intra-molecular Hydrogen bond: A bond that is formed within the molecule.
Consequences of Hydrogen bonding:
- They exist as associated molecules.
- Boiling points of compound having H-bond are higher as they have extra bonds in them.
- They generally exist as liquids.
Hydrogen bonding in ice and water
The structure of Ice is given below :
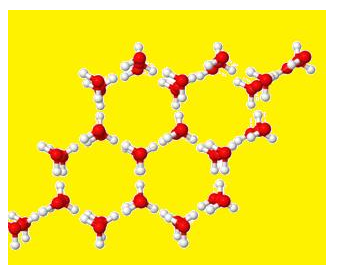
- In it each oxygen atom is linked to 4 Hydrogen.
- Two Hydrogen are linked by covalent bond and 2 Hydrogen by Hydrogen bonds.
- It forms network and space between is hollow.
- That is why; the ice is lighter due to hollow space.
- Therefore, ice floats on water.
- As we know, ice has high density so it should sink
- But, in actual practice it does not sink.
- Due to presence of hydrogen bonding in it, this makes the structure of ice hollow.
- Therefore, density decreases.
Water has maximum density at 4 degree Celsius:
This is seen because at zero degrees Celsius the water is ice. Now, if we start increasing its temperature gradually, we see that the cage of ice starts breaking. Due to it, hollow space starts getting filled. H-bonds break therefore, density increases.
At 4 degree Celsius, kinetic energy of molecules starts increasing and they start moving away due to which density decreases.