Carbon monoxide (CO)
Preparation
- It is prepared from incomplete combustion of carbon as given in reaction below :
C + ½ O2 –>CO
Carbon oxygen carbon monoxide
- Heating Methanoic acid in presence of sulphuric acid at temperature 473-1273 k.
HCOOH + H2SO4— >CO + H2O
Methanoic acid sulphuric acid carbon monoxide and water
- Commercial preparation: Heating Coke with water at high temperature.
C + H2O –> CO + H2
Carbon water syn gas
If we mix carbon in air :
C + O2+ 4N2 –> CO + N2
carbon oxygen nitrogen carbon monoxide and nitrogen gas
Properties
- It is colourless and odourless.
- It is insoluble in water.
- It is highly toxic in nature as it combines with Haemoglobin,to form defective complex .As a result, the oxygen carrying capacity of RBC gets reduced.
- It burns in air to form carbon dioxide.
Uses
- It acts as reducing agent in extraction of metals.
- It reacts with certain metals to form Metal carbonyls.
- It forms synthesis gas that has industrial advantage.
- It forms producer gas that has industrial advantage.
Carbon dioxide (CO2)
Preparation
- From complete combustion of Carbon and Carbon containing fuels in excess of air:
C + O2 — >CO2
carbon oxygen carbondioxide
CH4+ O2 –.>CO2 + H2O
Methane carbon dioxide and water
Properties
- It is colourless and odourless.
- It is soluble in water.
- It is non supporter of combustion.
- It combines with water to for Carbonic acid (weak dibasic acid).
Uses
- It forms carbonic acid which undergo dissociation as :
This H2CO3 and HCO3– helps in maintaining pH of blood between 7.26 to 7.42.
- Carbon dioxide is used by plants for photosynthesis: If the percentage of this gas increases in atmosphere, it has adverse affect like green house effect, which further results in ecological imbalance .
- Dry ice (solid form of carbon dioxide) is used as refrigerant for ice creams and frozen food.
- Gaseous carbon dioxide is used in carbonated soft drinks.
- Carbon dioxide is used in fire extinguisher as it is non supporter of combustion.
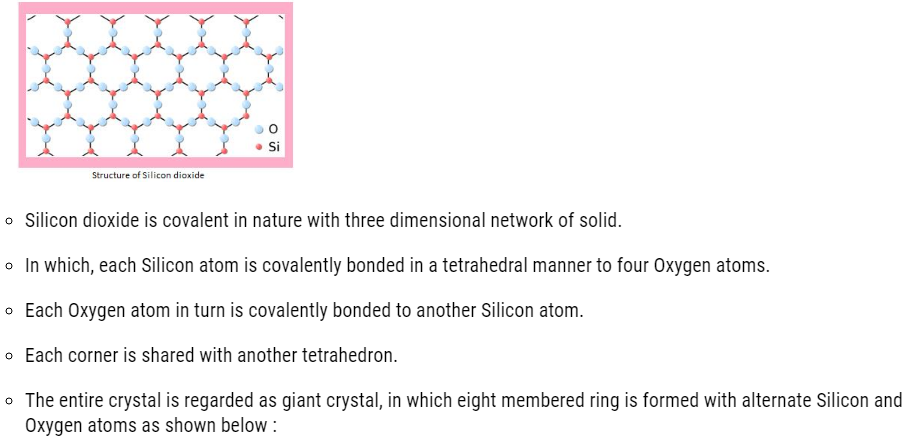
Silicon dioxide (SiO2)
About 95% of earth crust is made up of silica and silicates .Silicon dioxide is commonly called as silica and it occurs in different forms :
Crystalline form of silica
- Quartz
- Cristobalite
- Tridymite
These forms are inter-convertible at suitable temperature.
Structure of Silicon dioxide
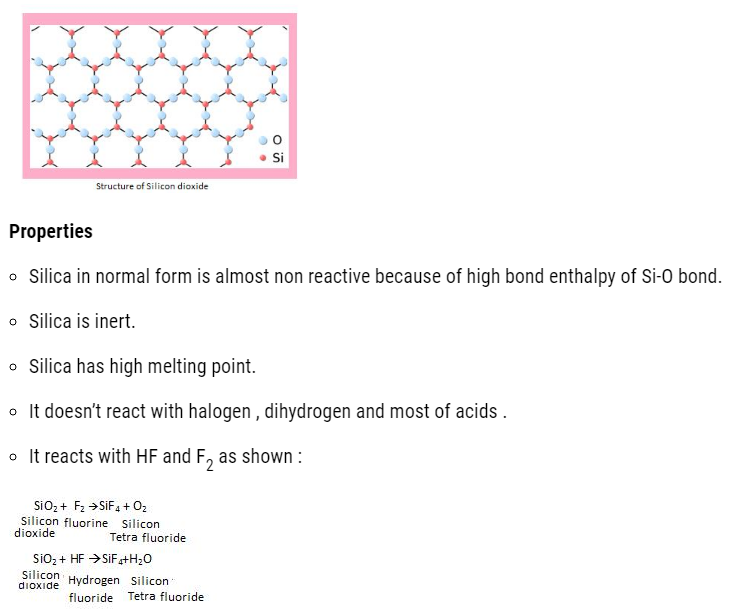
- This silicon tetra fluoride can easily dissolves in HF to form H2SiF6 (hydro-Fluoro-silicic acid ).
- Due to formation of this compound, the SiO2 present in glass gets dissolved. That is the reason the hydrogen fluoride can’t be stored in glass bottles, instead it is stored in containers made of Cu, Ni+ traces of Fe alloy .
- Quartz is used as piezoelectric material.
Uses
- Silicon dioxide is used as catalyst in petroleum industry.
- When (NH4)2CoCl4 is added to silica gel , it act as a humidity detector .Since ,it is blue when dry but turns pink when hydrated .
Silicones
- They are synthetic organo-silicon compounds containing repeated R2SiO units held by Si-O-Si linkages.
- These compounds have the general formula (R2SiO)n where R is methyl or aryl group .
Preparation
- The methyl chloride reacts with Silicon in presence of Copper at temperature 573k.
- As a result, we get different types of methyl substituted cholrosilane of formulas :MeSiCl3, MeSiCl2, Me3SiCl and also Me4
CH3Cl + Si –> (CH3)2SiCl2
methyl chloride dichloromethylsilane
(CH3)2SiCl2 + H2O –> (CH3)2Si(OH)2
Dichloromethylsilane
- If we carry out hydrolysis of dichlorodi-methylsilane followed by polymerization we get , straight chain polymers
Properties
- Silicones with :
- Short chains – oily liquids
- Medium chain- viscous oils, jellies and greases
- Long chains – rubbery elastomers and resins .
- They are chemically inert, resistant to oxidation and thermal decomposition.
- Silicones are surrounded with non polar alkyl group that are water repelling in nature.
- They are heat resistant and possess high dielectric constant.
Uses
- They are used in making water proof papers, wool ,textile, wood etc by coating them with thin film of silicones.
- They are used as electric insulators.
- They are used as lubricants at high as well as at low temperature , as there is very little change in their viscosity with temperature.
- They are used in surgical implants.

Zeolites
- They are widely used as catalyst in petrochemical industries for cracking of hydrocarbons.
- In them basically the Silicon atoms in three dimensional structures is replaced by Aluminum ions.
- As a result, the overall structure carries the negative charge .
- To balance this negative charge some cations like sodium ion etc are added in the structure.
For example:
- ZSM-5 a type of zeolite converts alcohols directly to gasoline.
- Hydrated zeolite is used as permutit in ion exchange method for softening of hard water.