Introduction
The reaction in which oxidation and reduction both occur simultaneously are called Redox reactions. Let us understand about oxidation and reduction. We have different concepts to understand oxidation and reduction.
Oxidation and Reduction
- In terms of Classical concept
Oxidation: Oxidation is defined as “addition of oxygen or any electronegative element and removal of hydrogen or any electropositive element”.

Please note the substance that undergo oxidation itself and reduce other is called reducing agent whereas the substance that undergo reduction itself and oxidize other is called oxidizing agent.
For example:- CuO + H2 –> Cu + H2O
Copper Oxide Hydrogen Copper Water
In reaction, Cu is undergoing reduction and oxidizing hydrogen. Therefore, Cu is oxidizing agent and H is reducing agent.
“As in this reaction both oxidation and reduction occur simultaneously, therefore reaction is called redox reaction.”
- In terms of Electron transfer concept
Oxidation: Is defined as loss of electrons that is:
M-electron –> M+
Metal Metal ion
Reduction: Is defined as gain of electrons that is given below.
N + electron –> N–
Non metal non metal ion
Let us explain it more with the help of example:
Let’s consider equation: H2 + O2 — > H2O
We can see in this example that H atom as going from neutral to a positive state in water, the O atom goes from zero state to dinegative state in water. There is an electron transfer from H to O and consequently H2 is oxidized and O2 is reduced .The charge transfer is only partial or we can say that it is electron shift rather than calling it as complete loss of electron by H and gain by O.
Another example of Redox reaction in terms of electron transfer:
Mg + H2 –> MgH2
Magnesium Hydrogen Magnesium Hydride
In this Magnesium is losing electrons, that is undergoing oxidation and hydrogen is gaining electrons therefore, it is undergoing reduction. Mg is Reducing agent in it and H is Oxidizing agent in it.
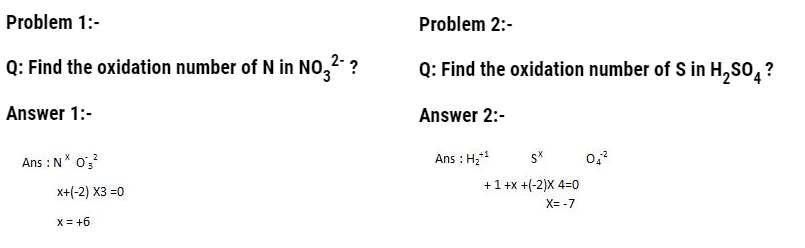
- In terms of oxidation number
Oxidation number: It is defined as “residual charge that is present on atom when the atom is in combined state with other atoms”.
Rules to assign and calculate oxidation number
- The oxidation number of atoms in their elemental state is taken as zero.
- The oxidation number of mono-atomic atoms like Na+ etc is taken as 1.
- The oxidation number of Hydrogen is +1 when present with non metals and -1 when present with metals.
- The oxidation number of oxygen is -2 in most of the compounds but in peroxides it is -1.
- The metals always have oxidation number in positive and non metal in negative when present together in ionic compounds.
- In compounds that have two atoms with different electro negativities, the oxidation number of more electronegative is taken as –ve and for less electronegative it is taken as positive. For example: In OF2 the oxidation number of oxygen will be in positive and oxidation of fluorine will be in negative.
- In neutral compounds, the sum of all oxidation numbers is equal to zero.
- In complex ions, the sum of oxidation states of all the atoms is equal to the charge present on the complex.
Oxidation: It is defined as increase in oxidation number of an element.
Mg + H2 –> Mg2+ H2
Magnesium Hydrogen MagnesiumHydride
In this, Magnesium oxidation number is increased from 0 to +2.Therefore, oxidation occurs.
Reduction: It is defined as decrease in oxidation number of an element.
For Example:-
Fe3+ + e– –> Fe2+
Ferric Ion Ferrous Ion
In this oxidation number of iron is reduced .Therefore, reduction occurs.
Redox reaction in terms of oxidation number
In this, Zinc is undergoing oxidation and act as reducing agent whereas Hydrogen is undergoing reduction and act as an oxidizing agent.
Zn0 + H+Cl– –> Zn2+ + 0H2
Zinc HydrogenChloride Zinc Chloride Hydrogen Gas