MORE QUESTIONS SOLVED ( States of Matter )
I. Very Short Answer Type Questions
Question 1. What is the value of the gas constant in SI units?
Answer: 8.314 JK-1 mol-1.
Question 2. Define boiling point of a liquid.
Answer: The temperature at which the vapour pressure of a liquid is equal to external pressure is called boiling point of liquid.
Question 3. What is SI unit of (i) Viscosity (ii) Surface tension?
Answer: (i) Unit of viscosity is Nsm-2
(ii) Unit of surface tension is Nm-1
Question 4. What is the effect of temperature on (i) surface tension and (ii) Viscosity?
Answer: (i) Surface tension decreases with increase of temperature.
(ii) Viscosity decreases with increase of temperature.
Question 5. What is the unit of coefficient of viscosity?
Ans. Poise.
Question 6. What do you understand by laminar flow of a liquid?
Answer: The type of flow in which there is regular gradation of velocity in passing from one layer to the next is called laminar flow.
Question 7. What do you mean by compressibility factor?
Answer: The deviation from ideal behaviour can be measured in terms of compressibility factor Z.
Z=PV/nRT
Question 8. What is Boyle Temperature?
Answer: The temperature at which a real gas obeys ideal gas law over an appreciable range of pressure, is called Boyle temperature or Boyle point.
Question 9. What is meant by elastic collision ?
Answer: Collision in which there is no loss of kinetic energy but there is transfer of energy, is called elastic collision.
Question 10. Define critical temperature of gas.
Answer: The temperature above which a gas cannot be liquefied.
Question 11. What are real gases ?
Answer: A gas which can deviate from ideal gas behaviour at higher pressure and lower temperature, is called a real gas.
Question 12. Define an ideal gas.
Answer: A gas that follows Boyle’s law, Charles’ law and Avogadro law strictly, is called an ideal gas.
Question 13. Name four properties of gases.
Answer:
- Gases, have no definite shape and no definite volume.
- There is no force of attraction existing between the molecules of gases.
- Gases are highly compressible.
- Gases’can mix evenly and can spread in whole space.
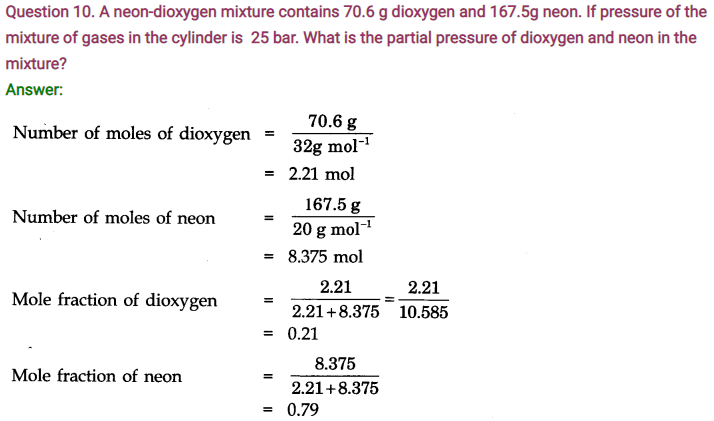
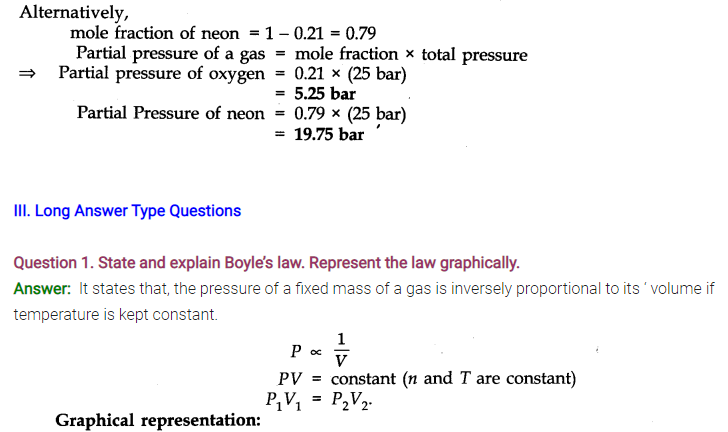
Question 14. State Dalton’s law of partial pressure.
Answer: Daltons’ Law states that, total pressure exerted by the mixture of non-reactive gases is equal to the sum of the partial pressures of individual gases.
Question 15. What do you mean by aqueous tension?
Answer: Pressure exerted by saturated water vapour is called aqueous tension.
Question 16. Give mathematical expression for ideal gas equation.
Answer: PV = nRT
Where R is called Gas constant.

Question 20. What is the effect of temperatures on the vapour pressure of a liquid?
Answer: Vapour pressure increases with rise in temperature.
Question 21. Why falling liquid drops are spherical?
Answer: Because of the property of surface tension, liquid tends to minimise its area.
II. Short Answer Type Questions
Question 1. A weather balloon has a volume of 175 dm3 when filled with hydrogen gas at a pressure of 1.0 bar. Calculate the volume of the balloon when it rises to a height where the atmospheric pressure is 0.8 bar. Assume that temperature is constant.
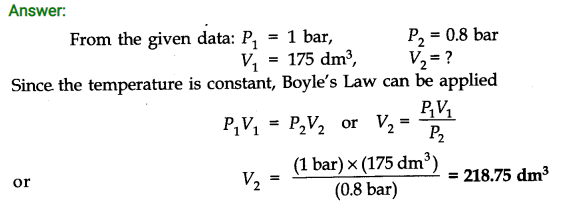







Question 9. (a) What do you mean by’Surface Tension’of a liquid?
(b) Explain the factors which can affect the surface tension of a liquid.
Answer: (a) Surface tension: It is defined as the force acting per unit length perpendicular to the line drawn on the surface. It’s unit is Nnr1.
(b) Surface tension of a liquid depends upon following factors.
(i) Temperature: Surface tension decreases with rise in temperature. As the temperature of the liquid increases, the average kinetic energy of the molecules increases. Thus, there is a decrease in intermolecular force of attraction which decreases the surface tension.
(ii) Nature of the liquid: Greater the magnitude of intermolecular forces of attraction in the liquid, greater will be the value of surface tension.

When n is the no. of moles present and ‘a’ and ‘V are known as van der Waals constants.
Significance of van der Waals constants
van der Waals constant ‘a’ : ‘a’ is related to the magnitude of the attractive forces among the molecules of a particular gas. Greater the value of V, more will be the attractive forces.
Unit of ‘a’ = L2 mol-2
van der Waals Constant ‘b’: ‘b’ determines the volume occupied by the gas molecules which depends upon size of molecule.
Unit of ‘b’ = L mol-1.
Question 3. What are ideal and real gases? Out of CO2 and NH3 gases, which is expected to show more deviation from the ideal gas behaviour?
Answer: Ideal Gas: A gas that follows Boyle’s law, Charles’ law and Avogadro law strictly, is called an ideal gas. It is assumed that intermolecular forces are not present between the molecules of an ideal gas.
Real Gases: Gases which deviate from ideal gas behaviour are known as real gases. NH3 is expected to show more deviation. Since NH3 is polar in nature and it can be liquified easily.
Question 4. State and explain Dalton’s law of partial pressures. Can we apply Dalton’s law of partial pressures to a mixture of carbon monoxide and oxygen?
Answer: Dalton’s law of partial pressure: When two or more non-reacting gases are enclosed in a vessel, the total pressure of the gaseous mixture is equal to the sum of the partial pressures that each gas will exert when enclosed separately in the same vessel at constant temperature.
P= P1 + P2 + P3
Where, P is the total pressure of the three gases A, B, and C enclosed in a container. P1 , P2 and P3 are the partial pressures of the three gases when enclosed separately in the same vessel at a given temperature one by one.
No, the law cannot be applied. Carbon monoxide and oxygen readily combine to form carbon dioxide. The law can be applied only to the non-reacting gases.