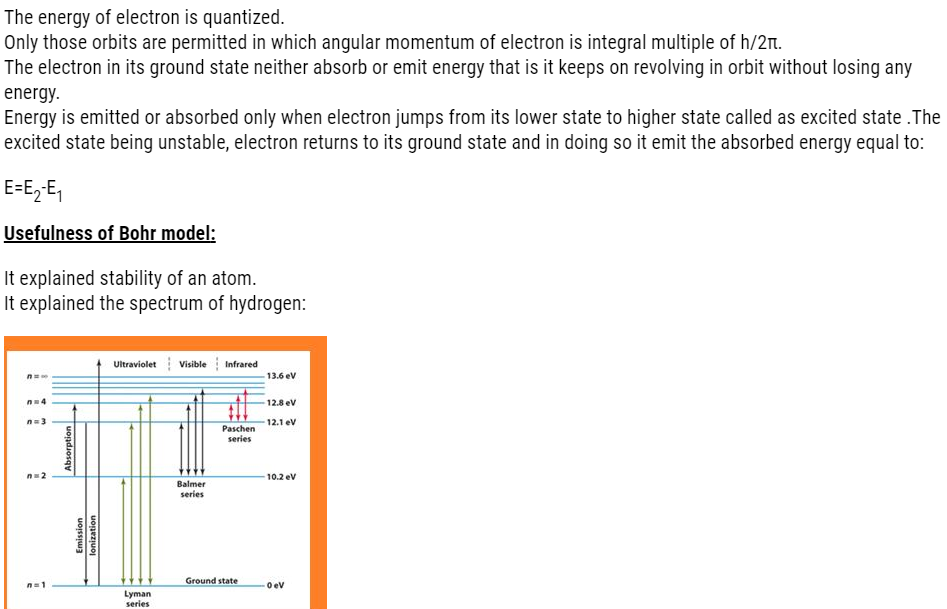
About Lesson
Planck’s quantum theory:
According to this theory:
- Energy emitted or absorbed is not continuous, but is in the form of packets called quanta .In terms of light it is called as photon.
- Each photon carries an energy which is directly proportional to the frequency of wavelength i.e. E depends upon v (nu).
- Or E=hv (where v is frequency)
- Value of h =6.634 x 10-34Jsec
- Energy associated with no of packets is given by:
E=nhv (where n is an integral multiple)
This formula can also be written as:
E = (nhc)/ (λ)
(Because we know frequency=speed of light/wavelength)
V = (c/ λ)
EXPLANATION OF BLACK BODY AND PHOTOELECTRIC EFFECT ON THE BASIS OF QUANTUM THEORY.
For the ejection, minimum frequency is required called threshold frequency .Let’s say light that falls, has energy equal to hv .Out of this h , a amount of h is used as binding energy and rest is given to electron as kinetic energy.
Mathematically


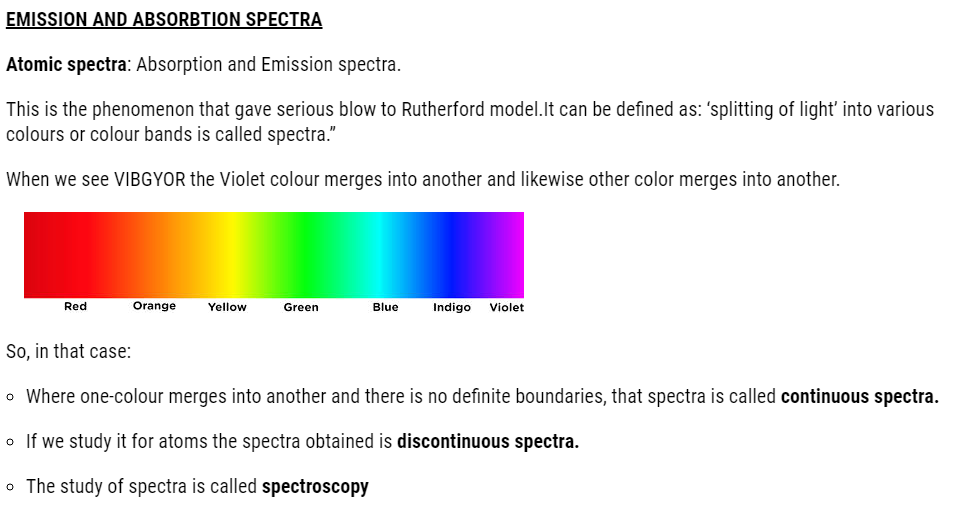


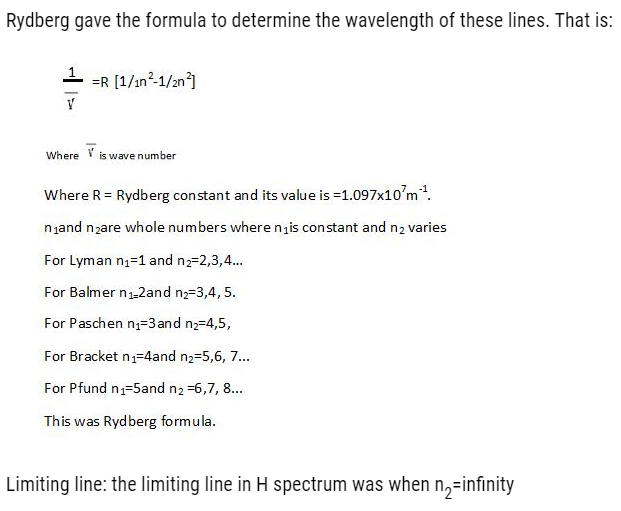
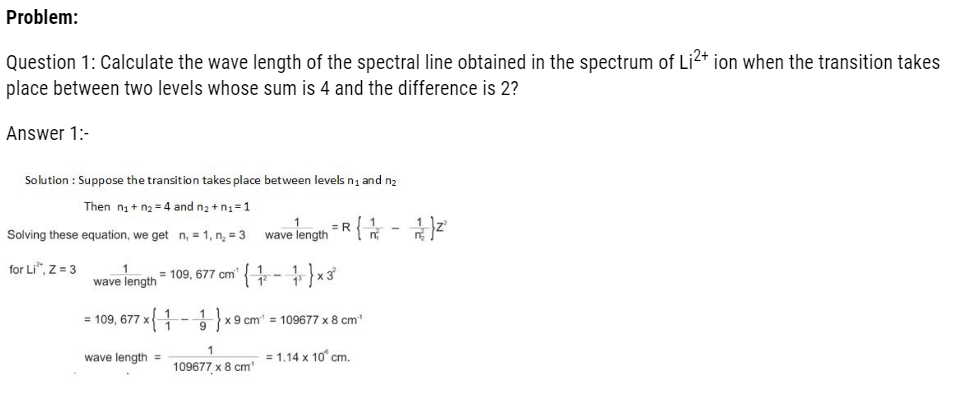
BOHR’S THEORY