NCERT TEXTBOOK QUESTIONS SOLVED
Question 1. Explain the formation of a chemical bond.
Answer: According to Kossel and Lewis, atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration. This can occur in two ways; by transfer of one or more electrons from one atom to other or by sharing of electrons between two or more atoms.
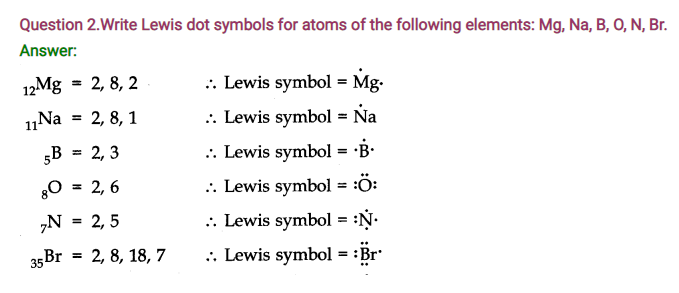
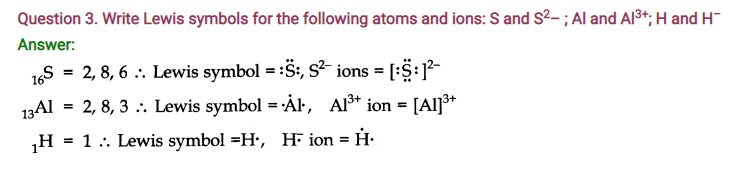
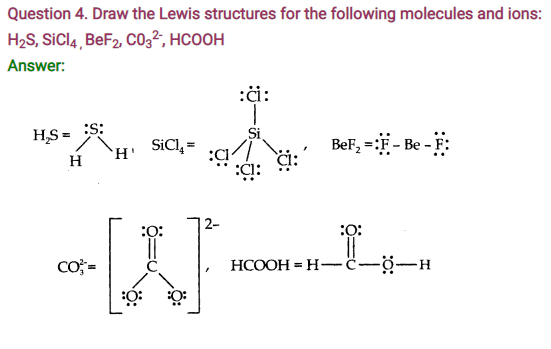
Question 5. Define Octet rule. Write its significance and limitations.
Answer: Octet rule: Atoms of elements combine with each other in order to complete their respective octets so as to acquire the stable gas configuration.
Significance: It helps to explain why different atoms combine with each other to form ionic compounds or covalent compounds.
Limitations of Octet rule:
- According to Octet rule, atoms take part in chemical combination to achieve the configuration of nearest noble gas elements. However, some of noble gas elements like Xenon have formed compounds with fluorine and oxygen. For example: XeF2, XeF4 etc.
Therefore, validity of the octet rule has been challenged. - This theory does not account for shape of molecules.
Question 6. Write the favourable factors for the formation of ionic bond.
Answer:
- Low ionization enthalpy of metal atoms
- High electron gain enthalpy of non-metal atoms
- High lattice enthalpy of compound formed.
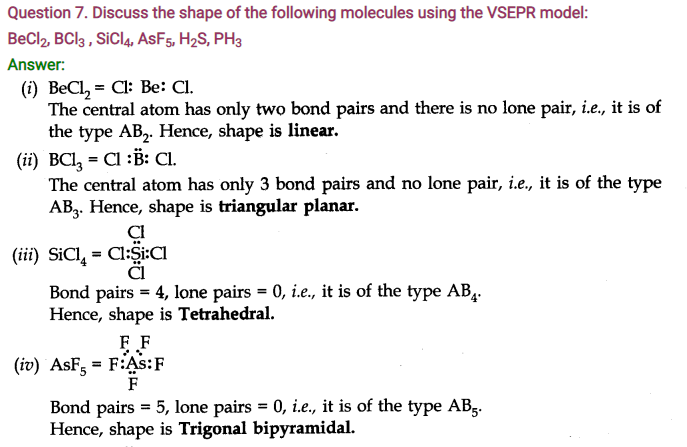

Question 9. How do you express the bond strength in terms of bond order?
Answer: Bond strength is directly proportional to the bond order. Greater the bond order, more is the bond strength.
Question 10. Define the bond-length.
Answer: Bond-length: It is the equilibrium distance between the nuclei of two bonded atoms in a molecule. Bond-lengths are measured by spectroscopic methods.

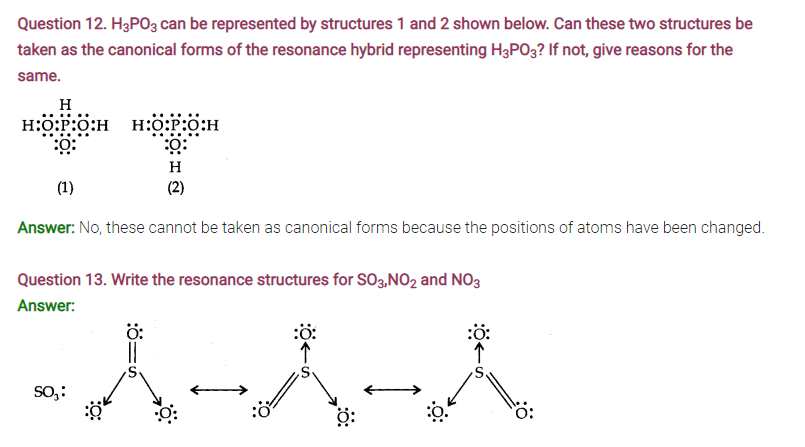

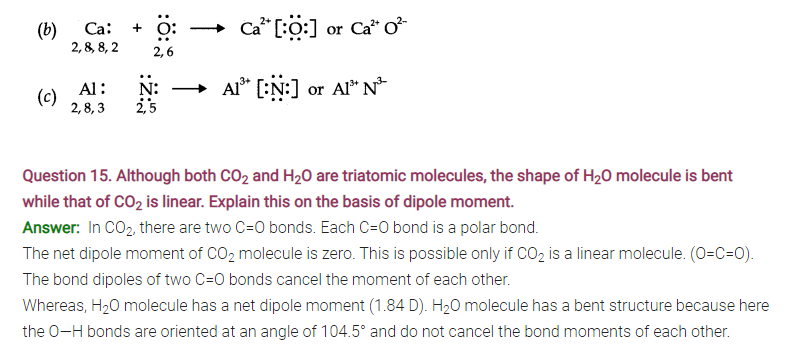
Question 16. Write the significance/applications of dipole moment.
Answer:
- In predicting the nature of the molecules: Molecules with specific dipole moments are polar in nature and those of zero dipole moments are non-polar in nature.
- In the determination of shapes of molecules.
- In calculating the percentage ionic character.
Question 17. Define electronegativity. How does it differ from electron gain enthalpy?
Answer: Electronegativity: Electronegativity is the tendency of an atom to attract shared pair of electrons. It is the property of bonded atom.
Whereas, electron gain enthalpy is the tendency of an atom to attract outside electron. It is the property of an isolated atom.
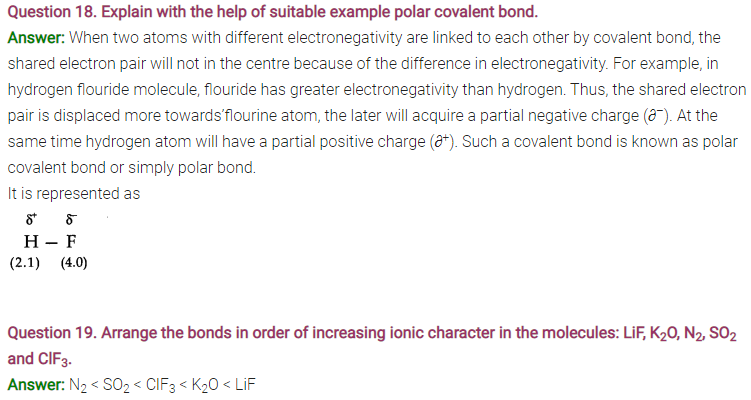
Question 21. Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the comers of the square and the C atom at its centre. Explain why CH4 is not square planar?
Answer: According to VSEPR theory, if CH4were square planar, the bond angle would be 90°. For tetrahedral structure, the bond angle is 109°28′. Therefore, in square planar structure, repulsion between bond pairs would be more and thus the stability will be less.
Question 22. Explain why BeH2 molecule has a zero dipole moment although the Be—H bonds are polar.
Answer: BeH2is a linear molecular (H—Be—H), the bond angle = 180°.
Be—H bonds are polar due to difference in their electronegativity but the bond polarities cancel each other. Thus, molecule has resultant dipole moment of zero.
Question 23. Which out of NH3 and NF3 has higher dipole moment and why?
Answer: In NH3 and NF3, the difference in electronegativity is nearly same but the dipole moment of NH3 = (1.46D) For Example, NH3 = (0.24D)
In NH3, the dipole moments of the three N—H bonds are in the same direction as the lone pair of electron. But in NF3, the dipole moments of the three N—F bonds are in the direction opposite to that of the lone pair. Therefore, the resultant dipole moment in NH3 is more than in NF3.
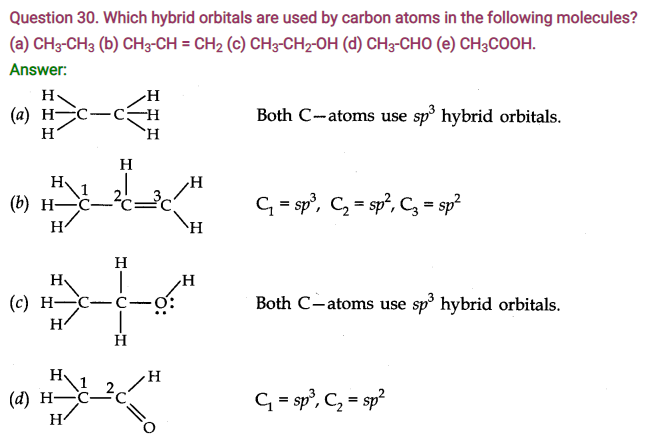
Question 24. What is meant by hybridisation of atomic orbitals? Describe the shapes of sp, sp2, sp3 hybrid orbitals.
Answer: Hybridisation: It is defined as the process of intermixing of atomic oribitals of slightly different energies to give rise to new hybridized orbitals having equivalent energy and identical shapes.
Shapes of Orbitals:
sp hybridisation: When one s-and one p-orbital, intermix then it is called sp-hybridisation. For example, in BeF2, Be atom undergoes sp-hybridisation. It has linear shape. Bond angle is 180°.
sp2 hybridisation: One s-and two p-orbitals get hybridised to form three equivalent hybrid orbitals. The three hybrid orbitals directed towards three corners of an equilateral triangle. It is, therefore, known as trigonal hybridisation.
sp3 hybridisation: One s-and three p-orbitals get hybridised to form four equivalent hybrid orbitals. These orbitals are directed towards the four corners of a regular tetrahedron.
Question 25. Describe the change in hybridisation (if any) of the Al atom in the following reaction. AlCl3 + Cl– ——> AlCl4- .
Answer: Electronic configuration of 13Al = 1s2 2s2 2p6 3s1 3px13py1
(excited state)
Hence, hybridisation will be SP2
In AlCl–4, the empty 3pz orbital is also involved. So, the hybridisation is sp3 and the shape is tetrahedral.
Question 26. Is there any change in the hybridisation ofB and N atoms as a result of the following reaction ? BF3 + NH3 ——-> F3 B.NH3
Answer: In BF3, B atom is sp2 hybridised. In NH3, N is sp3 hybridised.
After the reaction, hybridisation of B changes from sp2 to sp3.
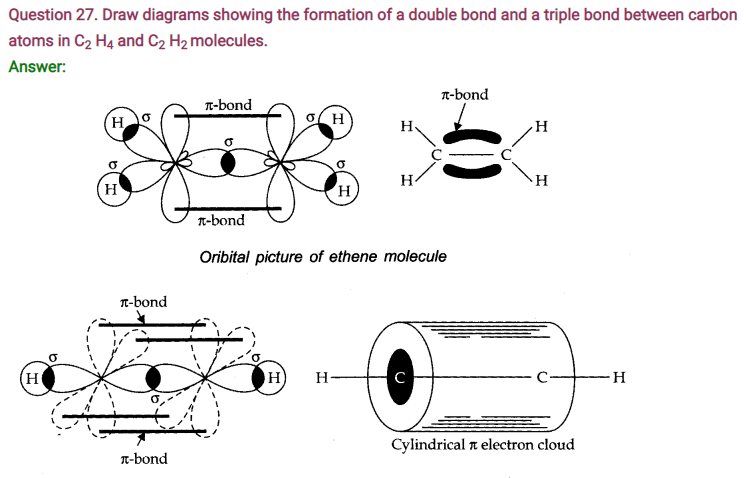


Question 33. Explain the formation of H2 molecule on the basis of valence bond theory.
Answer: Let us consider the combination between atoms of hydrogen HA and HB and eA and eB be their respective electrons.
As they tend to come closer, two different forces operate between the nucleus and the electron of the other and vice versa. The nuclei of the atoms as well as their electrons repel each other. Energy is needed to overcome the force of repulsion. Although the number of new attractive and repulsive forces is the same, but the magnitude of the attractive forces is more. Thus, when two hydrogen atoms approach each other, the overall potential energy of the system decreases. Thus, a stable molecule of hydrogen is formed.
Question 34. Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Answer:
- The combining atomic orbitals should have comparable energies.
For example, Is orbital of one atom can combine with Is atomic orbital of another atom, 2s can combine with 2s. - The combining atomic orbitals must have proper orientations. So that they are able to overlap to a considerable extent.
- The extent of overlapping should be large.