Physical properties of H2
- It is colourless.
- It is odourless.
- It is tasteless.
- Its vapor density = 1 (lighter than air).
- It is non – poisonous.
- It is solubility in H2O is very low.
Chemical Properties –
- It is chemicaly inert at room temperature.
- Its chemical nature depends upon Bond dissocitaion energy of ( H – H)bond.
- The Bond dissociation energy is high because of comparable sizes . Due to small size ,it requires high temperature to break the bond that is 2000k or more than that . At 5000k it gets 95.5% dissociates.
- Although the Bond dissociation energy is high for H – H, but still Hydrogen is reactive . It has only 1 valence electron so, it combines or we can say react to attain 2electrons is its
It Can attain stability by :
- Burning in presence of O2 – It reacts with oxygen to form water and the reaction is exothermic.
2H2 + O2 –> H2O + Energy
Hydrogen Oxygen Water
- Reaction with halogens : it react with halogens to form respectives halides as shown below :
H2 + Cl2 –> 2HCl
Hydrogen Chlorine HydrogenChloride
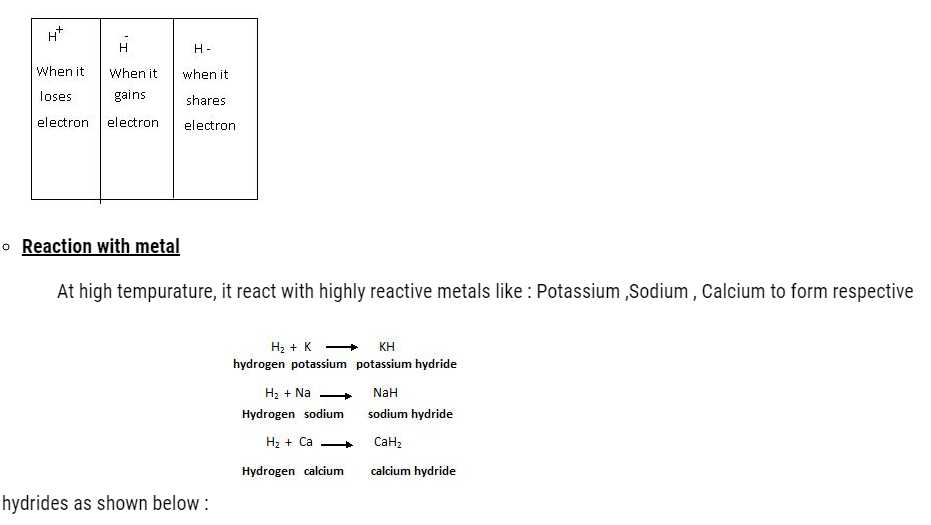
- Reaction with N-metal –
It reacts with many non metals to form hydrides. Like with sulphur it forms sulphides ,with Oxygen it forms oxides ,with Flourine it forms flourides etc as shown below :
1. H2 + S –> H2S
Hydrogen Sulphur HydrogenSulphide
2. 2H2 + O2 –> H2O
Hydrogen Oxygen Water
3. H2 + F2 –> HF
Hydrogen Fluroide HydrogenFluroide
4. N2 + 3H2 –> 2NH3(Haber’s Process) (In the presence of catalyst)
Nitrogen Hydrogen Ammonia
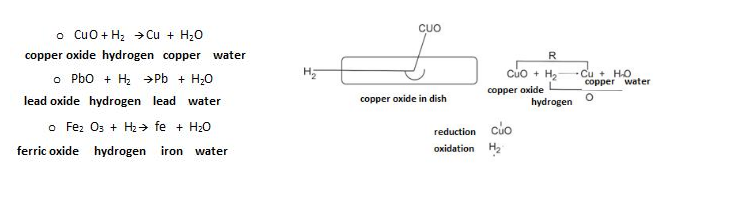
- H2 as Reducing agent –
As we have discussed in the introduction of hydrogen ,that it act as a reduvcing agent so, here we have certains examples as given below :
- Hydrogenation of Oils –
It is the Process of converting vegetable oil into vanaspati ghee by adding H2 in presence of catalyst nickel.
Vegetable Oil —> Vanaspati Ghee(In presence of Ni)
Uses of H2 –
- It is used as rocket fuel because of its high calorific value.
- OxyHydrogenflame is used for welding.
- It helps in synthesis of NH3(ammonia) , HNO3 (niric acid),HCl(hydrochloric acid) etc.
- It helps in the synthesis of vanspati ghee.
- It is a reducing agent .