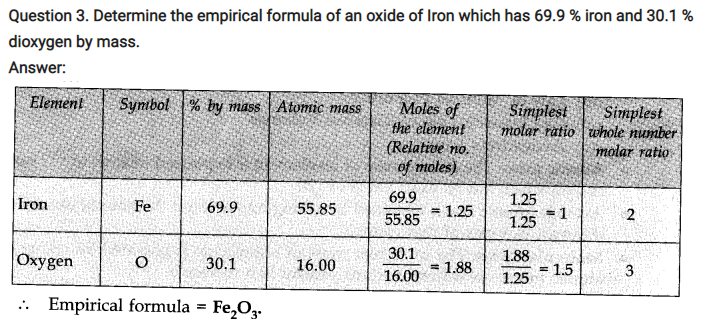
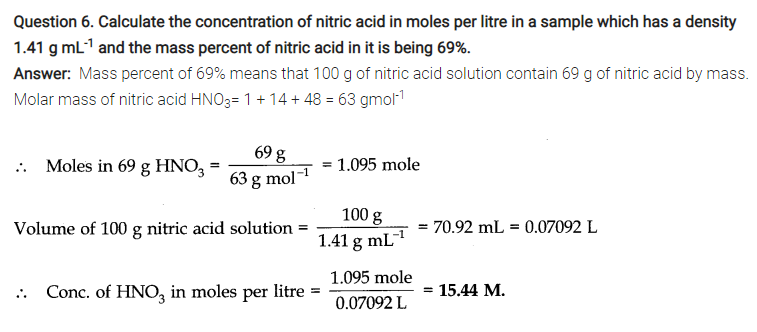
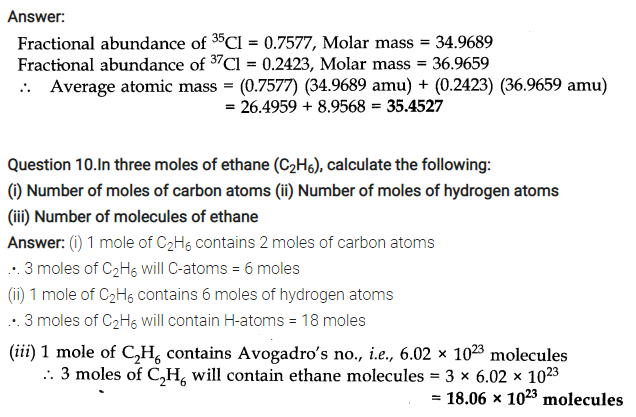
NCERT TEXTBOOK QUESTIONS SOLVED
Question 1. Calculate the molecular mass of the following:
(i) H20(ii) C02(iii) CH4
Answer: (i) Molecular mass of H2O = 2(1.008 amu) + 16.00 amu=18.016 amu
(ii) Molecular mass of CO2= 12.01 amu + 2 x 16.00 amu = 44.01 amu
(iii) Molecular mass of CH4= 12.01 amu + 4 (1.008 amu) = 16.042 amu


(ii)
Hence, O2 is the limiting reagent.
∵ 32 g O2 reacts with C to produce 44 g of CO2
∵ 16 g O2 reacts with C to produce
(iii)
∵ 64 g O2 reacts with C to produce 88 g of CO2
∵ 16 g O2 reacts with C to produce =

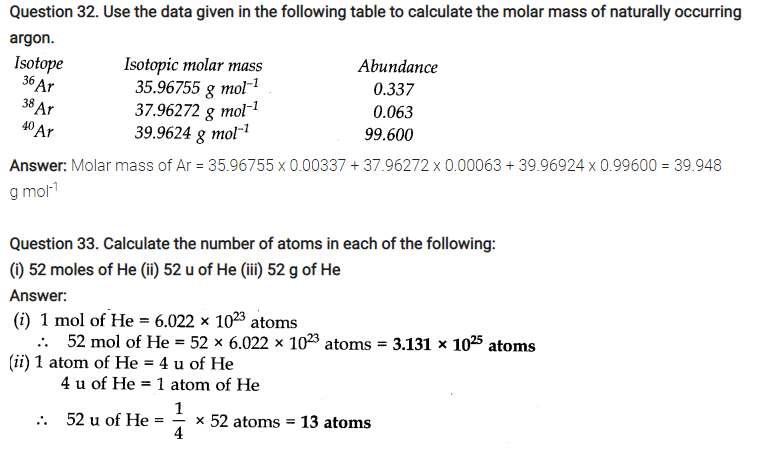
Question 7. How much copper can be obtained from 100 g of copper sulphate (CuSO4 )? (Atomic mass of Cu= 63.5 amu)
Answer: 1 mole of CuS04 contains 1 mole (1 g atom) of Cu
Molar mass of CuS04= 63.5 + 32 + 4 x 16 = 159.5 g mol-1
Thus, Cu that can be obtained from 159.5 g of CuS04 = 63.5 g

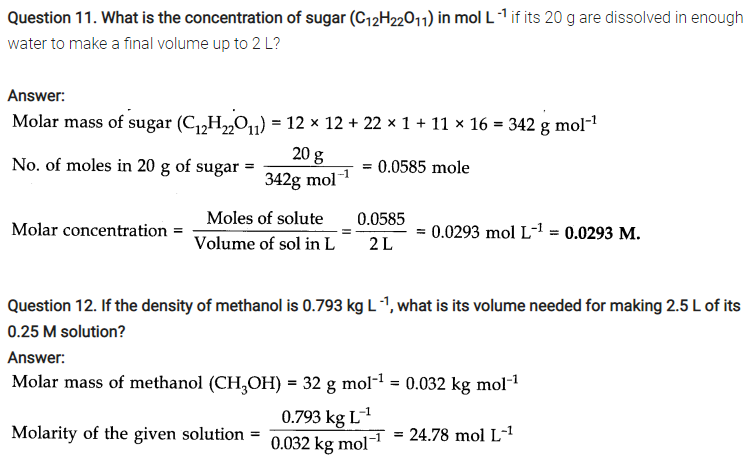


Question 23. In the reaction, A + B2——> AB2, identify the limiting reagent, if any, in the following mixtures
(i) 300 atoms of A + 200 molecules ofB
(ii) 2 mol A + 3 mol B
(iii) 100 atoms of A + 100 molecules ofB
(iv) 5 mol A + 2.5 mol B
(v) 2.5 mol A + 5 mol B
Answer: (i) According to the given reaction, 1 atom of A reacts with 1 molecule of B
.•. 200 molecules of B will react with 200 atoms of A and 100 atoms of A will be
left unreacted. Hence, B is the limiting reagent while A is the excess reagent.
(ii) According to the given reaction, 1 mol of A reacts with 1 mol of B
.•. 2 mol of A will react with 2 mol of B. Hence, A is the limiting reactant.
(iii) No limiting reagent.
(iv) 2.5 mol of B will react with 2.5 mol of A. Hence, B is the limiting reagent.
(v) 2.5 mol of A will react with 2.5 mol of B. Hence, A is the limiting reagent.

Question 25. How are 0.50 mol Na2C03 and 0.50 M Na2C03 different?
Answer: Molar mass of Na2C03= 2 x 23 + 12 + 3 x 16 = 106g mol-1 0.50 mol Na2C03 means 0.50 x 105 g = 53 g 0. 50 M Na2C03 means 0.50 mol, i.e., 53 g Na2C03 are present in 1 litre of the solution.
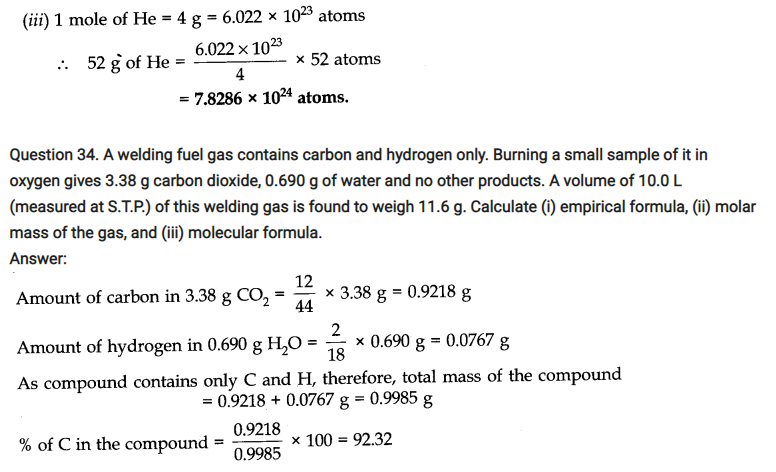
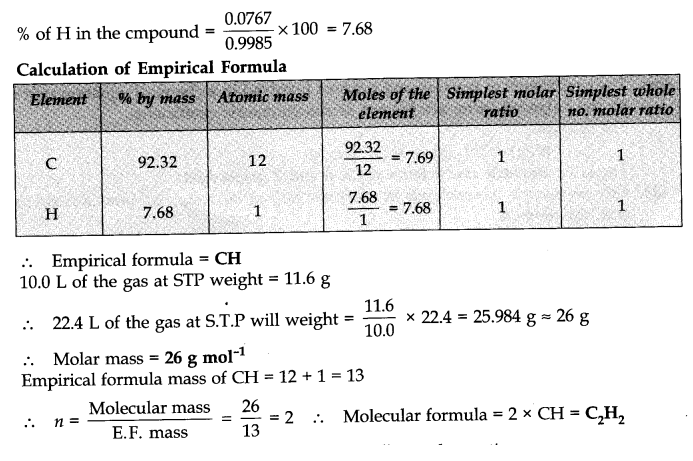
Question 26. If ten volumes of dihydrogen gas reacts with five volumes of dioxygen gas, how many volumes of water vapour could be produced?
Answer: H2 and 02 react according to the equation
2H2(g) + 02 (g) ——>2H2O (g)
Thus, 2 volumes of H2 react with 1 volume of 02 to produce 2 volumes of water vapour. Hence, 10 volumes of H2 will react completely with 5 volumes of 02 to produce 10 volumes of water vapour.






MORE QUESTIONS SOLVED
I.Very Short Answer Type Questions
Question 1. What is the SI unit of molarity?
Answer: SI unit of molarity = mol dm-3
Question 2. What do you understand by stoichiometric coefficients in a chemical equation?
Answer: The coefficients of reactant and product involved in a chemical equation represented by the balanced form, are known as stoichiometric coefficients.
For example, N2(g) + 3H2(g) ———–> 2 NH3(g)
The stoichiometric coefficients are 1, 3 and 2 respectively.
Question 3. Give an example of a molecule in which the ratio of the molecular formula is six times the empirical formula.
Answer: The compound is glucose. Its molecular formula is C6H12O6, while empirical formula is CH2O.
Question 4. What is an atom according to Dalton’s atomic theory?
Answer: According to Dalton’s atomic theory, an atom is the ultimate particle of matter which cannot be further divided.
Question 5. Why air is not always regarded as homogeneous mixture?
Answer: This is due to the presence of dust particles.
Question 6. Define the term ‘unit’ of measurement.
Answer: It is defined as the standard of reference chosen to measure a physical quantity.
Question 7. Define law of conservation of mass.
Answer: It states that matter can neither be created nor destroyed.
Question 8. How is empirical formula of a compound related to its molecular formula?
Answer: Molecular formula = (Empirical formula)n where n is positive integer.
Question 9. How many oxygen atoms are there in 18 g of water?
Answer: Molar mass of water is 18 g/mol.
Number of oxygen atoms is 18 g of water = 6.02 x 1023
Question 10. Name two factors that introduce uncertainty into measured figures.
Answer: (i) Reliability of measuring instrument.
(ii) Skill of the person making the measurement.
Question 11. State Avogadro’s law.
Answer: Equal volumes of all gases under the conditions of same temperature and pressure contain the same number of molecules.
Question 12. How are 0.5 ml of NaOH differents from 0.5 M of NaOH?
Answer: 0.5 ml of NaOH means 0.5 mole (20.0 g) of NaOH, 0.5M of NaOH means that 0.5 mole (20.0g) of NaOH are dissolved in 1L of its solution.
Question 13. What is one a.m.u. or one ‘u’?
Answer: 1 a.m.u. or 1 u = 1/12 th mass of an atom of carbon 12.
Question 14. What is the number of significant figures in 1.050 x 104?
Answer: Four.