About Lesson
Introduction
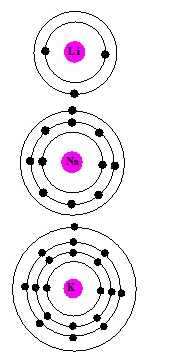
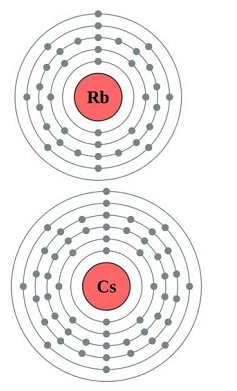
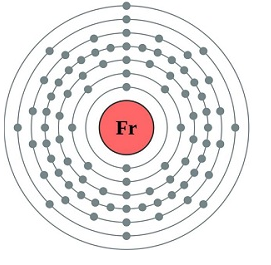
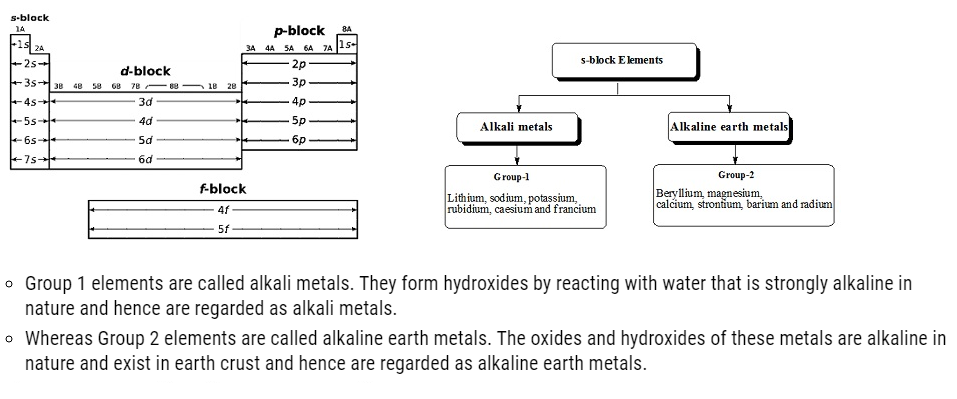
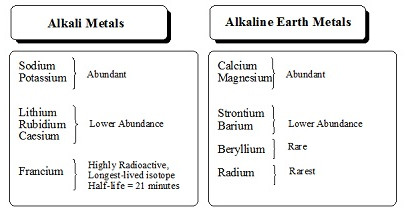
The elements in which the last electron enters the outermost s-orbital are called s-block elements. s-block has two groups (1 and 2).
Diagonal Relationship
- The similarity in the properties of definite pairs of diagonally adjacent elements in the second and third periods of the periodic table is called diagonal relationship.
- In s-block elements Lithium is the first element of group 1 whereas Beryllium is the first elementsof Group 2.
- Some of their properties do not match with the properties exhibited by other elements of their group.
- Instead their properties resemble the properties of the second element of the following group due to the similarity in ionic sizes and /orcharge/radius ratio of the elements.
- Consequently lithium and magnesium have similar properties whereas Beryllium and Aluminium exhibit similar properties.
- This relation is called diagonal relationship.



- Both the elements are harder and lighter than the other elements.
- Both of them react slowly with
- The oxides and hydroxides of both the elements are less soluble.
- The hydroxides of both the elements decompose on heating.
- Both the elements form nitrides by combining directly with nitrogen.
6 Li+ N2 (g) = 2 Li3N
3 Mg(s) + N2 (g) = Mg3N2(s)
- Both Li2O and MgO do not produce their superoxide by combining with excess oxygen.
- Chlorides of both lithium (LiCl) and magnesium (MgCl2) are soluble in ethanol, are deliquescent and crystallize as hydrates.
LiCl·2H2O
MgCl2·8H2O

Atomic and Ionic Radii of alkali metals
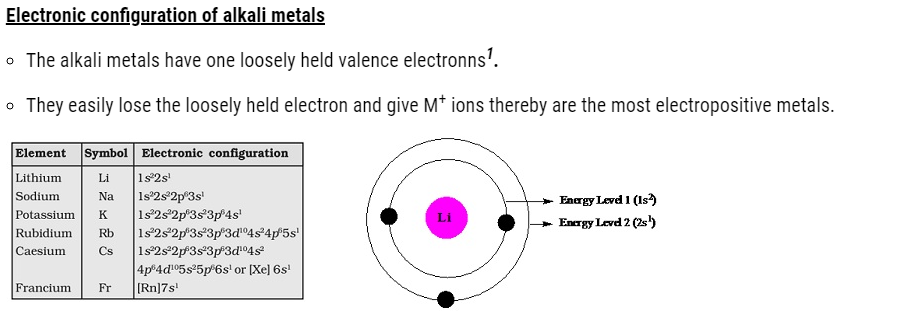
- In Group 1 the atom of the atomic and ionic radii increases down the group.
- Increase in atomic number increases the size of the atom of the elements.