Alkanes can be classified as:
- Primary alkanes(10)
- Secondary alkanes (20)
- Tertiary alkanes (30)
- Primary alkane: Alkane in which a Carbon atom attached to one or none alkyl group is = 10
CH3-CH3 (ethane)
10
2. Secondary Alkane: In which a carbon is linked to two alkyl groups is =20
Example in propane:- CH3-CH2-CH3
20
3.Tertiary Alkane: Alkane in which a carbon atom is linked to three alkyl groups is =30

Preparation of alkanes
- Naturally, they are synthesised by decomposition of plants and waste matter.
- In laboratory: They are prepared from :
- From unsaturated hydrocarbons
- From alkyl halides
- By reduction of alkyl halides
- By use of Grignard reagent
- From carboxylic acids
(a) From unsaturated Hydrocarbons:-The method involved is by hydrogenation that is addition of H2. It is also called as reduction reaction.
The general reaction involved:

A Special reaction occurs where we use Raney Ni which is actually activated nickel (alloy of 50 % Ni, 50 % Al). But the reaction is assigned a special name that is Sabatier Sanderson Reduction. The reaction is given below:

Similarly this reaction can be carried out for alkynes and the same name is assigned:

(b)From Alkyl halides (RX)
These alkyl halides are formed by replacing Hydrogen by halogen (X).
Example: RCl or CH3Cl etc
The methods that can be used for preparing alkanes by using alkyl halides are:
- Wurtz reaction
- Reduction of alkyl halide
- By use of Grignard reagent
Wurtz reaction: in this two molecules of alkyl halide react with sodium in presence of dry ether as shown below:
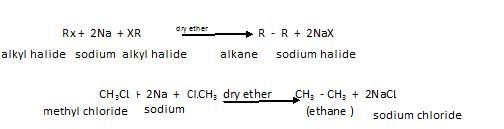
Please note the dry ether is used so as to keep sodium dry so that it does not catch fire in moist conditions.
Another example:

Limitation of this reaction: is that we need to take same alkyl halides otherwise we get mixtures of alkanes and due to almost same boiling points etc, they are difficult to separate. Another limitation of this is that by this method we can prepare alkanes only with even number of Carbon atoms.
(c)Reduction of alkyl halides: – It can be done by using various Reducing agents like:
- With Zn and HCl
CH3CH2Cl + H2 à CH3CH3 + HCl
(Ethyl Chloride) (Ethane) Hydrogen chloride
In this the Zn and HCl both react and produce nascent Hydrogen to carry out reduction.
(Zn/HCl)
CH3CH2X + 2H2 –> CH3CH3 + HX
(Ethyl halide) (Ethane) (Hydrogen Halide)
- Pt or Pd can be used as reducing agent
(Pt/Pd)
CH3CH2Br + H2 –> CH3CH3 + HBr
(Ethyl Bromide) (Ethane) (Hydrogen Bromide)
- With use of reducing agent HI in presence of Red Phosphorous.
(Red P)
CH3CH2X + HI –> CH3CH3 + I2 + H2
(Ethyl Halide) (Ethane) (Iodine) Hydrogen Gas
The Role of Red P is to remove I2 from reaction so as to get maximum yield of alkane because it is a reversible reaction.
Red P + I2 –> PI3
Phosphorous Iodide
- By use of Reducing agent Zn and Cu couple

With use of very strong Reducing agent like LiAlH4 ,NaBH4
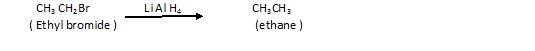
(d) By use of Grignard reagent (RMgX)

(e) From Carboxylic acids (RCOOH)
- By Decarboxylation: It is removal of of CO2 In this first carboxylic acid, is made to react with sodium metal then followed by its reaction with sodium hydroxide to get the desired alkane.
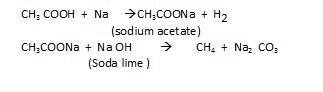
Kolbe’s electrolysis Reaction: In this electric current is passed through metal carboxylic salt

Reduction of carboxylic acid: In this carboxylic acid is reduced in presence of hydrogen iodide as shown :
