Conformational Isomerism
It arises due to free rotation around C – C bond. We can define it as:
Definition: The different arrangement of atoms in space which can be obtained due to rotation about
C – C bond are called Conformation. It is not totally free but there is energy barrier of 120kJ/mol that has to be overcome. This is called torsion strain that exist between two forms.
Conformations of ethane: There are two projections.
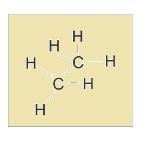
- Sawhorse projection: In this H and C are bonded to each other and is represented in form of lines.
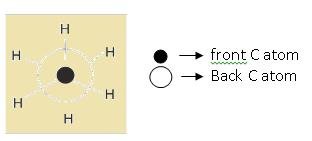
Newman projection: In this carbon is represented by drawing circles and hydrogen are represented by drawing lines as shown.
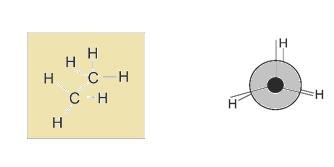
The two form of new man projection are: eclipsed and staggered
- Staggered: In this hydrogen are far apart from each other

- Eclipsed: this hydrogen are just at back of each other
When staggered is rotated by 600 we get eclipsed form .In this: 600 -torsional angle .Torsional angle is angle by which one form is rotated to get the other form.
Torsional strain: It is the repulsive force that acts and give differnt stability of different forms .
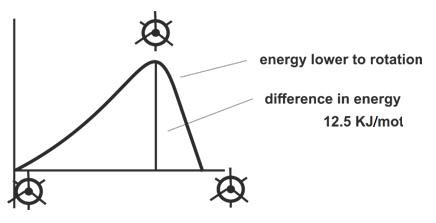
The difference in their energy is given above and this is easily overcome and they keep inter converting.
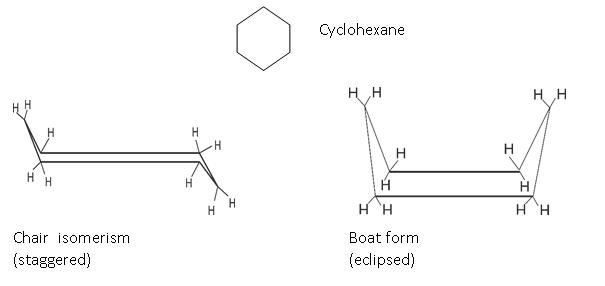
In this cyclohexane: We have two forms that are shown: