NCERT Solutions for Class 11 Chemistry Chapter 14 Very Short Answer Type Questions
Question 1. What do you mean by primary and secondary pollutants of the air?
Answer: Primary pollutants are those which after their formation remains as it was before e.g., NO. Secondary pollutants are formed as a reaction with primary pollutants e.g., PAN (peroxyacyl nitrates).
Question 2. What is the name of the compound formed when CO combines with blood?
Answer: Carboxyhaemoglobin.
Question 3. How are NO and NO2 formed in the atmosphere?
Answer: NO is formed due to the reaction between N2 and O2 during lightning or by the combustion of fossil fuels.NO is oxidised to form NO2.
Question 4. What is chlorosis?
Answer: Slowdown of process of formation of chlorophyll in plants with the presence of SO2 is called chlorosis.
Question 5. Which zone is known as ozonosphere?
Answer: Stratosphere.
Question 6. Which main gases is responsible for damage in ozone layer?
Answer: NO and CFCs
Question 7. What is the nature of classical smog?
Answer: Reducing.
Question 8. Name the acids which are responsible for acid rain?
Answer: H2SO4, HNO3 and HCl.
Question 9. What is BOD?
Answer: The amount of oxygen consumed by micro organism in decomposing organic wastes of sewage water is called BOD (Biochemical Oxygen Demand).
Question 10. What do you mean by viable and non-viable particulates?
Answer: Viable particulates are microorganisms like bacteria, fungi, moulds, algae etc. Non- viable particulates are formed by the disintegration of bigger size particles or by the condensation of water vapour, e.g., mist, smoke, fume and dust.
Question 11. What is siltation?
Answer: Mixing of soil or rock particles in water is called siltation.
Question 12. What is the composition of London Smog?
Answer: London Smog consists H2SO4 deposited on the particulates suspended in the atmosphere.
Question 13. List out the gases which are considered as major source of air pollution.
Answer: Carbon monoxide (CO), sulphur dioxide (SO2) and oxides of nitrogen (NO2).
Question 14. Why is acid rain considered as threat to Taj Mahal ?
Answer: Acids present in acid rain can react with marble (CaCO3) and damage the monument.
Question 15. Give one example of organic herbicide.
Answer: Triazines.
Question 16. What are pesticides ?
Answer: Pesticides are the substances used to kill unwanted pests. For example, DDT.
Question 17. What is PAN stands for?
Answer: It is peroxyacetyl nitrate.
Question 18. Give the examples of insecticides.
Answer: DDT, BHC.
Question 19. Which gas was mainly responsible for Bhopal gas tragedy?
Answer: Methyl isocyanate.
Question 20. What is meant by polar vortex?
Answer: A tight whirlpool of wind formed in the stratosphere which surrounds Antarctica is called polar vortex.
Question 21. What should be the tolerable limit of F ions in drinking water?
Answer: 1 ppm or 1 mg dm-3.
NCERT Solutions for Class 11 Chemistry Chapter 14 Short Answer Questions ( Environmental Chemistry )
Question 1. How did carbon monoxide act as a poison for human beings?
Answer: Carbon monoxide is poisonous because it combines with hemoglobin of R.B.C. to form carboxyhemoglobin as.
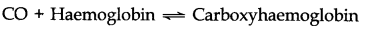
It inhibits the transport of oxygen to different parts of the body. Thus the body becomes oxygen-starved.
Question 2. What is ‘Acid Rain’? How is it harmful to the environment?
Answer: Acid rain is the rain water mixed with small amount of sulphuric acid, nitric acid along with hydrochloric acid which are formed from the oxides of sulphur and nitrogen present in air as pollutants. It has a pH of 4-5.
Harmful effects of Acid Rain
- It is toxic to vegetation and aquatic life.
- It damages buildings and statues. Taj Mahal has been damaged by acid rain.
- Acid rain corrodes water conducting pipes resulting in the leaching of heavy metals such as iron, lead, etc., to the drinking water.
Question 3. What is photochemical smog? What are its effects? How can it be controlled?
Answer: This is a type of smog formed in warm, dry and sunny climate. They are formed when sunlight is absorbed by SO2, oxides of nitrogen and hydrocarbons.
They act as oxidising agents.
Effects of photochemical smog
- They produce irritation in the eyes and also in respiratory system.
- They can damage many materials such as metals, stones, building materials etc.
- NO2 present gives a brown colour to the photochemical smog which reduces visibility.
- It is harmful to fabrics, crops and ornamental plants.
Control of photochemical smog
- By using catalytic converters in automobiles.
- By spraying certain compounds into atmosphere which generate free radicals that can easily combine with the free radicals that initiate the reaction forming toxic compounds of photochemical smog.
- Certain plants such as pinus, juniparus, pyrus could be helpul in this matter.
Question 4. What do you mean by greenhouse effect? What is the role of CO2 in the greenhouse effect?
Answer: It is the phenomenon in which earth’s atmosphere traps the heat from the sun and prevents it from escaping in outer space. Gases such as CO2, methane, ozone, CFCs are believed to be responsible for this effect.Heat from the sun after being absorbed by the earth is absorbed by CO2 and then radiated back to the earth. Thus making the environment of the earth warm.
Question 5. (a) Define eutrophication and pneumoconiosis.
(b) Write difference between photochemical and classical smog.
Answer: (a) Eutrophication: When the growth of algaes increases in the surface of water, dissolved oxygen in water is reduced. This phenomenon is known as eutrophication. (Due to this growth of fish gets inhibited).
Pneumoconiosis: It is a disease which irritates lungs. It causes scarring or fibrosis of the lung.
(b)
| Photochemical smog | Classical smog |
| (i) It is formed as a result of photochemical decomposition of nitrogen dioxide and chemical reactions involving hydrocarbons. (ii) It takes place during dry warm season in presence of sunlight. (iii) It is oxidising in nature. |
(i) It is formed due to condensation of SO, vapours on particles of carbon in cold climate. (ii) It is generally formed during winter when there is severe cold. (iii) It is reducing in nature. |
NCERT Solutions for Class 11 Chemistry Chapter 14 Multiple Choice Questions
Question 1. Which of the following acid is most abundant in acid rain?
(a) HCl (b) HNO3 (c) H2SO4 (d)Organic acid
Question 2. Which of the following causes less pollution?
(a) NOx (b) SOx (c) CO2 (d) CxHy
Question 3. Besides CO2, the other greenhouse gas is
(a) Ar (b) N2 (C) O2 (d) CH4
Question 4. BOD is a measure of
(a) Organic pollutant in water (b) Inorganic pollutant in water
(c) Particulate matter in water (d) All of the above
Question 5. The gas which reacts with hemoglobin in blood is
(a) CO (b) SO2 (c) CO2 (d) NO2
Question 6. Ozone depletion is mainly due to
(a) HCFs (b) CFCs (c) CH3Br (d)all of the above
Question 7. The pollutant released in Bhopal gas tragedy was
(a) Ammonia (b) Mustard gas (c) Nitrous oxide (d) Methyl isocyanate
Question 8. Which of the following will increase the BOD of water supply?
(a) O3 (b) C2H5OH (C) H2O (d) CO2
Answer: 1.(c) 2. (c) 3. (d) 4. (a) 5. (a) 6.(b) 7. (d) 8. (c)
NCERT Solutions for Class 11 Chemistry Chapter 14 Hots Questions
Question 1. What is meant by PCBs?
Answer: PCBs are polychlorinated biphenyls. They are contaminants of water. They are used as fluids in transformers and capacitors.
Question 2. What is the compound formed when CO combines with blood?
Answer: When CO combines with blood, the following reaction occurs forming carboxyhaemoglobin:-
HB + CO —> (HBCO) Carboxyhaemoglobin
Question 3. Give three examples in which green chemistry has been applied.
Answer:
- In dry-cleaning, use of liquefied CO2 in place of tetrachloroethene (Cl2C = CCl2).
- In bleaching of paper using H2O2 in place of chlorine.
- In the manufacture of chemicals like ethanal using environment-friendly chemicals and conditions.