Chemical properties of alkenes
It shows mainly two types of reactions:
- Electrophilic addition reaction :
- Oxidation reaction
- Electrophilic addition reactions
The basic mechanism involved in it is shown below:
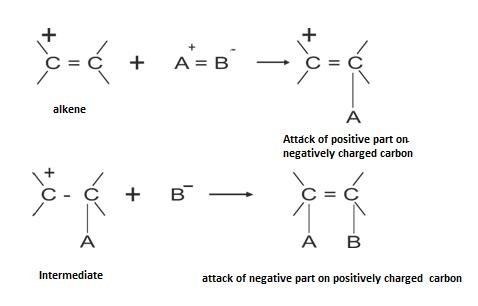
The more the ease of reaction, more stable is the intermediates i.e. carbocation.
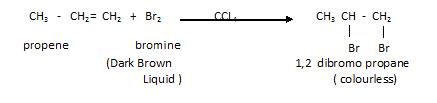
- Addition of halogen

Order of reactivity of halogens towards this reaction is:
F2> Cl2> Br2> I2
- In case of addition of fluorine, the reaction occurs so violently that it leads to explosion, as it is an exothermic reaction.
- In case of addition of iodine, the reaction take place under special conditions as the reaction is reversible.
- This reaction is also considered as a test for un-saturation (alkenes and alkynes): bromine water test. This test is given below.
(Test for un-saturation)
- Addition of halogen acids (HX)
The order of reactivity towards this reaction is:

The HI has weak bond because of incomparable size, that is the reason its reactivity is high towards this reaction.
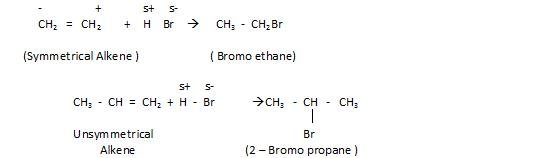
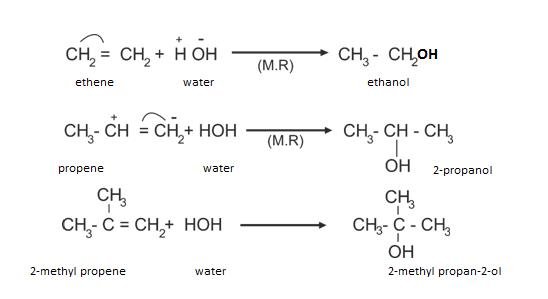
In case of unsymmetrical alkenes, Markovnikov’s rule is followed.
- Markovnikov’s rule: According to this, addition of any group in unsymmetrical alkene, the negative part of the reagent will attach itself to the Carbon atom carrying lesser number of Hydrogen and Hydrogen goes to the Carbon with maximum number of hydrogen.
- But if Reaction occurs in presence of peroxide like benzoyl peroxide, The rule that is followed is Anti-Markovnikov’s Rule or Kharasch effect (and this valid only for HBr ) .
- Anti Markonikovs Rule :According to it the negative part attaches to the carbon atom with maximum number of hydrogen and positive part attaches to the carbon atom with lesser hydrogen .
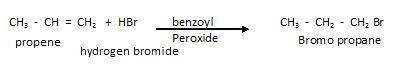
- Mechanism involved is free radical mechanism as shown below :
- Chain initiation step:
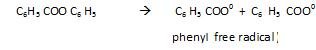
This radical is unstable therefore it decomposes to form C6 H5* (Phenyl free radical) and CO2.
- Chain Propagation step:
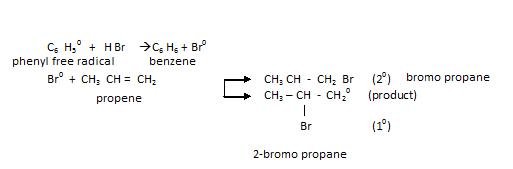
We know 20 free radical more stable therefore, the preferred product is going to be that which helps in yielding 20 free radical.
Please note:
- Markonikov’s Rule is for all attacking agents.
- Anti-Markonikov’s Rule is only for HBr (due to thermodynamic reasons).
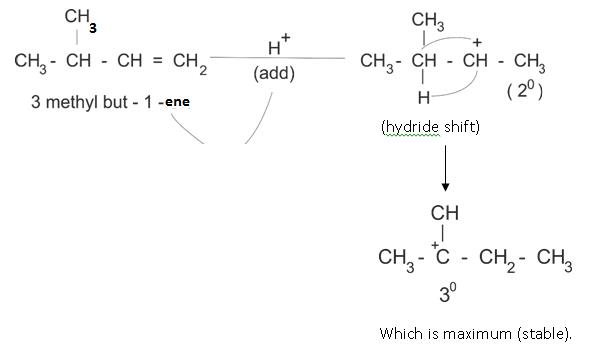
Rearrangement of Carbocation – Hydride shift
(d)Addition of H2O
- Oxidation Reactions
The oxidation can occur in different ways:
Combustion
Complete oxidation: In this CO2, H2O, heat and light is produced
Incomplete combustion: In this CO, H2O and heat and light are given out.
Controlled oxidation: It occurs in different ways:
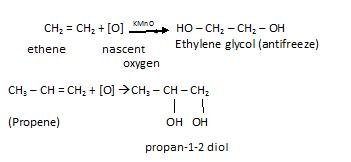
- Oxidation with (cold & dilute) KMnO4: Whenever oxidation of alkenes occurs in presence of strong oxidising agent like potassium permanganate. In it hydroxylation occurs and as a result alcohols (diols) are formed.
For example:
- Oxidation with hot and concentrated KMnO4: When alkenes react with hot and concentrated potassium permanganate, the cleavage occur across double bond :
For example:
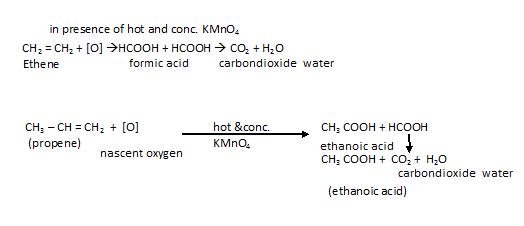
Please note: If there is no Hydrogen atom with carbon connected by double bond than ketone is formed and If there is H with carbon, then carboxylic acid is formed.
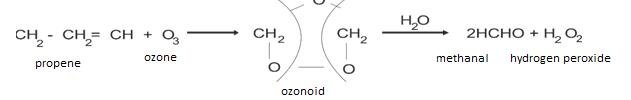
- Oxidation with ozone: It is also called as ozono- lyses.